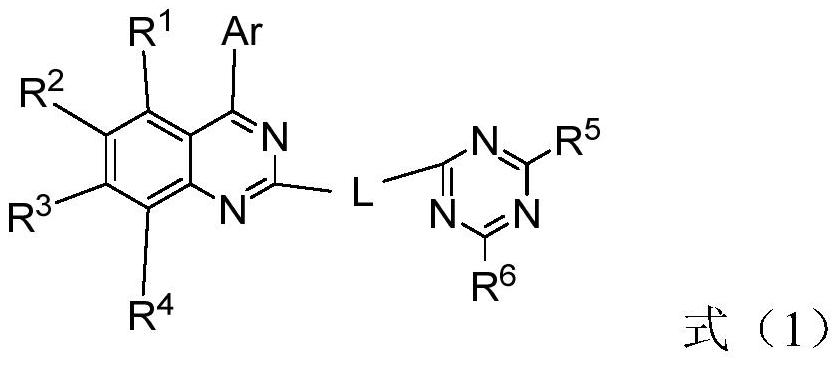
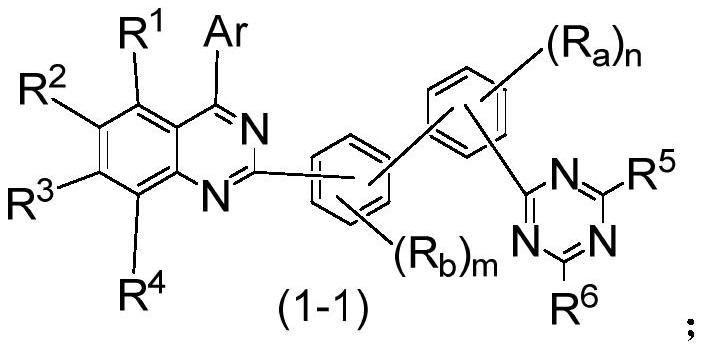
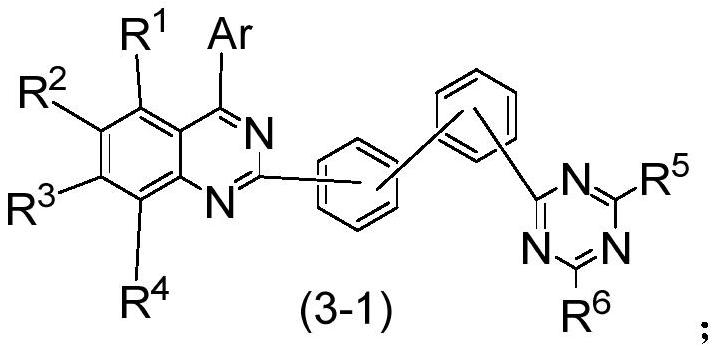
Compound, application thereof and organic electroluminescence device
A compound and unsubstituted technology, applied in the field of organic electroluminescence, can solve the problems that the types and properties of electron transport materials cannot fully meet the needs of devices, so as to improve the efficiency of electron injection and migration, strong ability to accept electrons, and high electron affinity. Harmony effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0137] Synthesis of compound C1:
[0138]
[0139] (1) Preparation of compound 1-2
[0140]Add raw materials 1-1 (38.7g, 100mmol), 3-chlorophenylboronic acid (15.6g, 100mmol), potassium carbonate (41.4g, 300mmol) and tetrakistriphenylphosphopalladium (1.15g, 1mmol) in the single-necked bottle, add Toluene 300mL, ethanol 100mL, water 60mL, under the protection of nitrogen, the reaction was refluxed overnight at 110°C. A solid precipitated out during the reaction. It was detected by thin layer chromatography (TLC) that the reaction of raw materials was completed, the reaction was stopped and cooled to room temperature, the precipitated solid was filtered, rinsed with water and ethanol respectively, and dried. The target compound 1-2 (38.5 g, yield 92%) was obtained.
[0141] (2) Preparation of compound 1-3
[0142] Compound 1-2 (33.5 g, 0.08 mol), pinacol borate (30.5 g, 0.12 mol) and potassium acetate (24 g, 0.24 mol) were added to a flask containing 1,4-dioxane (300 mL)...
preparation example 2
[0146] Synthesis of compound C17:
[0147]
[0148] (1) Preparation of compound 2-1
[0149] Add raw materials 4-bromobenzenecyano (18.1g, 100mmol), 4-chlorophenylboronic acid (15.6g, 100mmol), potassium carbonate (41.4g, 300mmol), tetrakistriphenylphosphopalladium (1.15g, 1mmol) in the single-necked bottle , add 300 mL of toluene, 100 mL of ethanol, and 60 mL of water, and react under reflux at 110° C. for 4 hours under the protection of nitrogen. It was detected by TLC that the reaction of the raw materials was complete, the reaction was stopped and cooled to room temperature, the liquid was separated, the organic phase was washed with water, dried, the solvent was removed under reduced pressure, purified by column chromatography, and dried. The target compound 2-1 (19.2 g, yield 90%) was obtained.
[0150] (2) Preparation of compound 2-2
[0151] Add compound 2-1 (17g, 0.08mol), pinacol borate (30.5g, 0.12mol) and potassium acetate (24g, 0.24mol) into a flask containi...
preparation example 3
[0157] Synthesis of compound C25:
[0158]
[0159] The difference between the specific synthesis steps and Preparation Example 1 is that the raw material 1-1 was replaced with the same amount of raw material 3-1 to obtain white solid compound C25, the calculated molecular weight: 665.25, and the measured value C / Z: 665.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com