Application of pharmaceutical composition in preparation of therapeutic drug for tumor insensitive to PD-1 antibody immunotherapy
A technology of PD-1 and immunotherapy, applied in the field of medicine, can solve the problem of insensitivity of antibody immunotherapy and achieve the effect of inhibiting tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
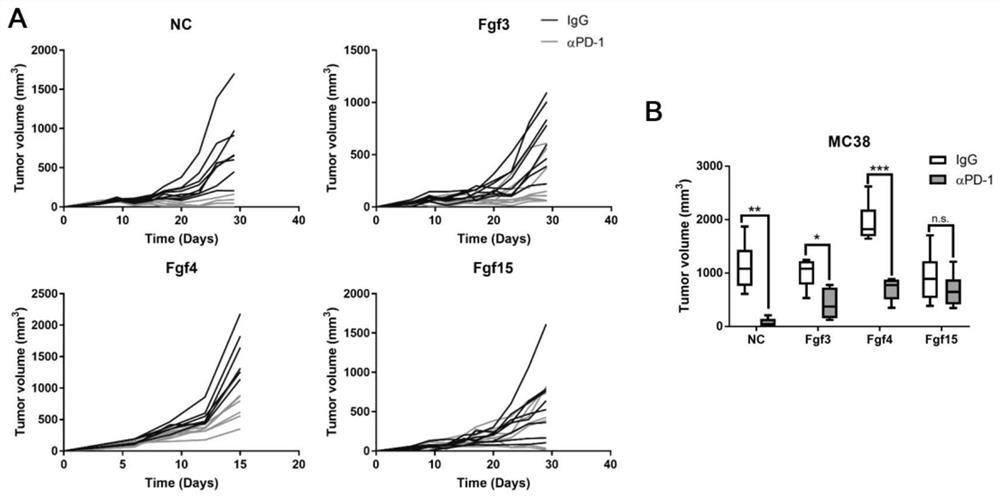
[0026] Example 1 The influence of key driver genes Fgf3, Fgf4, and Fgf15 in the chromosome 11q13.3 segment on PD-1 antibody treatment
[0027] 1. Experimental materials:
[0028] (1) Tumor cell line: In this experiment, the classic mouse tumor cell line MC38 used in the basic research of immunotherapy was used.
[0029] (2) Experimental mice: C57BL / 6J mice.
[0030] (3) Drug: murine PD-1 antibody, clone number G4C2.
[0031] (4) Lentiviral transfection system: murine Fgf3, Fgf4, Fgf15 overexpression lentivirus and control empty vector virus.
[0032] 2. Experimental group:
[0033] Tumor cells overexpressing the empty vector and tumor cells overexpressing mouse Fgf3, Fgf4, and Fgf15 were subcutaneously formed in C57BL / 6J mice, and they were divided into two groups:
[0034] (1) Control group: given IgG control treatment;
[0035] (2) Treatment group: treated with murine PD-1 antibody.
[0036] 3. Experimental steps:
[0037] (1) Construction of cell lines stably overexp...
Embodiment 2
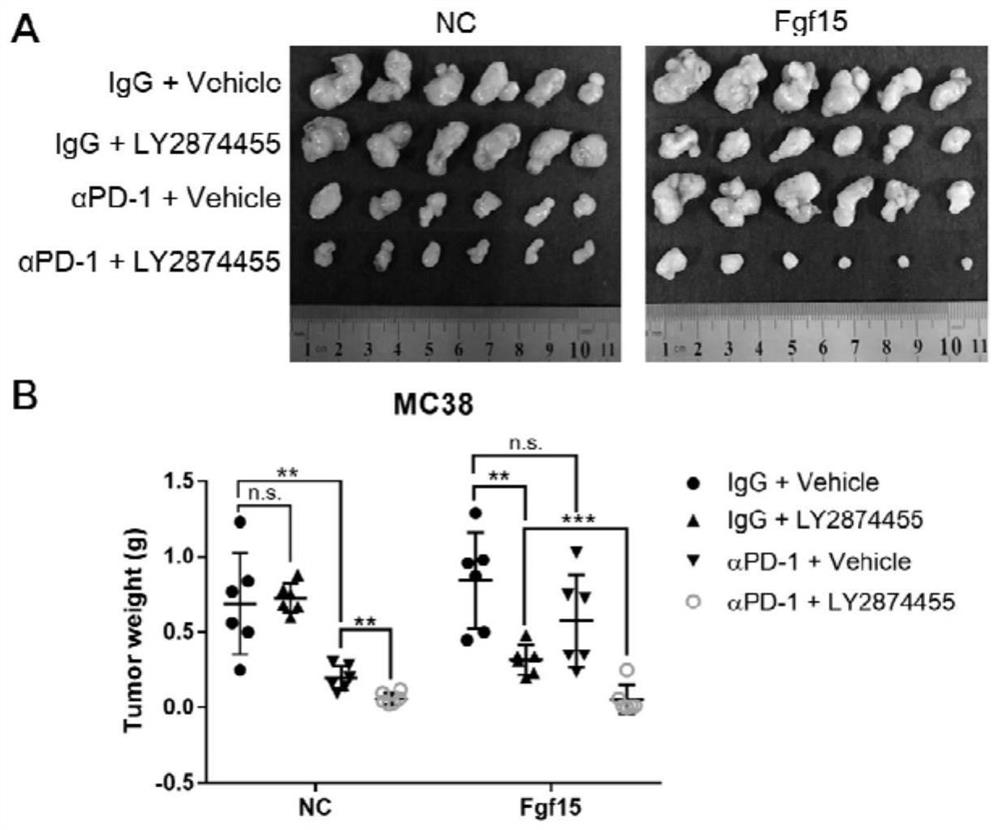
[0043] Example 2 PD-1 antibody and LY2874455 have a combined therapeutic effect on tumors with overexpressed Fgf15
[0044] 1. Experimental materials:
[0045] (1) Tumor cell line: Same as Example 1.
[0046] (2) Experimental mice: same as in Example 1.
[0047] (3) Drug: murine PD-1 antibody, clone number G4C2; LY2874455.
[0048] (4) Lentiviral transfection system: murine Fgf15 overexpression lentivirus and control empty vector virus.
[0049] 2. Experimental group:
[0050] Tumor cells overexpressing the empty vector and tumor cells overexpressing mouse Fgf15 were subcutaneously formed into tumors in C57BL / 6J mice, and each was divided into 4 groups:
[0051] (1) Control group: given IgG control treatment;
[0052] (2) Treatment group 1: treated with murine PD-1 antibody;
[0053] (2) Treatment group 2: LY2874455 treatment;
[0054] (2) Treatment group 3: treated with mouse PD-1 antibody combined with LY2874455;
[0055] 3. Experimental steps:
[0056] (1) Construc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com