Bovine infectious rhinotracheitis virus and mycoplasma bovis dual inactivated vaccine and its preparation method and suspension mdbk cells used
A technology of rhinotracheitis virus and double inactivated vaccine, applied in the directions of virus/phage, virus antigen components, biochemical equipment and methods, etc., can solve the problem of affecting the effect of vaccine use, not involving the prevention of Mycoplasma bovis, and increasing animal stress and other problems, to achieve the effect of saving economic costs, high sensitivity, and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078]Example 1 Acquisition of MDBK cells capable of suspension culture of bovine infectious rhinotracheitis virus
[0079] 1. Screening of serum-free suspension medium
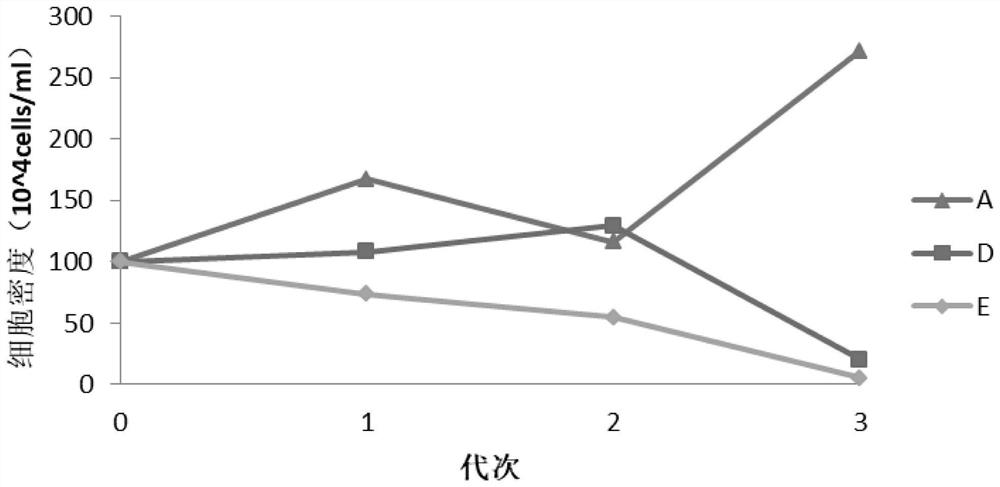
[0080] Set up 5 treatments in 5 suspension media:
[0081] Treatment A: Suspension medium was prepared by using the ST cell suspension medium of Eternal Life Sciences.
[0082] Treatment B: The suspension medium was prepared by using the Universal Suspension Cell Culture Medium of Yishengke.
[0083] Treatment C: Suspension medium was prepared using MDBK cell serum-free medium.
[0084] Treatment D: Suspension medium was prepared using CD MDBK 249.
[0085] Treatment E: Suspension medium was configured with OPM-MDBK SFM1 DPM.
[0086] For each treatment, the above serum-free medium and DMEM basal medium were mixed at a volume ratio of 1:1, and FBS and PS (penicillin-streptomycin mixture) were added to obtain a medium called suspension medium. The volume percentage of FBS in the base is 3%, the content of...
Embodiment 2
[0116] Example 2 The acquisition of the double inactivated vaccine of bovine infectious rhinotracheitis virus and mycoplasma bovis
[0117] 1. Preparation of IBRV antigen in suspension culture
[0118] 1.1 Preparation of vaccine strains
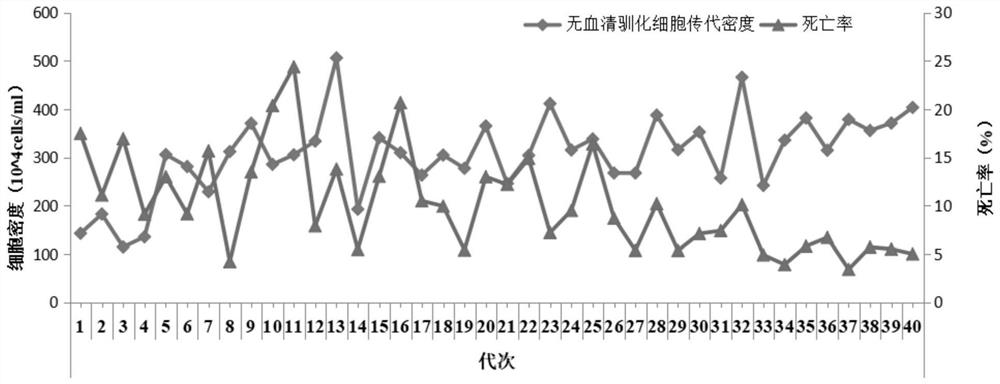
[0119] Suspended MDBK cells from Example 1 at an initial density of 1 × 10 6cells / ml, cultured in 125ml shake flasks (25ml per flask), after culturing for 48h, centrifuged at 300g (or 1000rpm) for 10min, using fresh serum-free suspension medium (preheated at 37°C for 30min) in 2.2.1 of Example 1 ) to dilute the cells to 4×10 6 cells / ml (25-30ml per flask), placed in a new cell shaker flask. The IBRV-SZH strain was inoculated into the shake flask of suspension cell culture at MOI=0.1 to carry out adaptive culture, and the shake flask was placed in 5% CO. 2 3. Culture on a shaker in a constant temperature incubator at 37°C, adjust the speed of the shaker to 100 rpm, and continuously cultivate for three generations, each generation for 48-72...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com