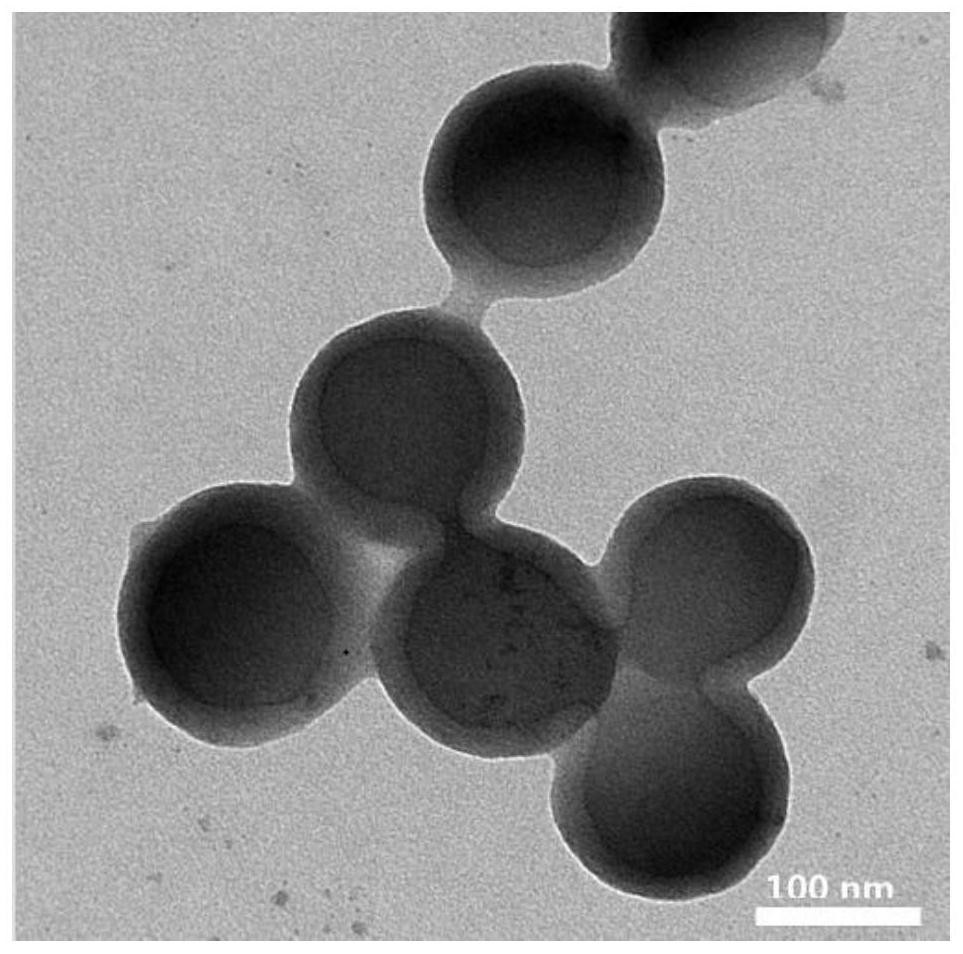
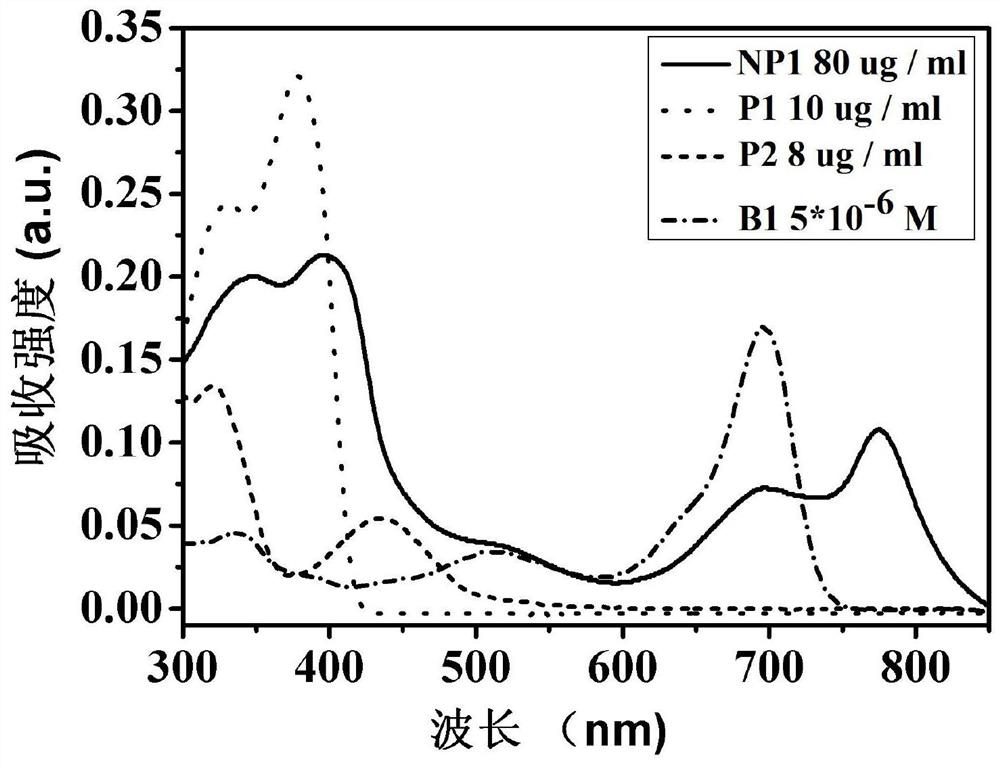
Nanoparticle releasing H2S under hypoxic condition as well as preparation method and application of nanoparticle
A nanoparticle, hydrogen sulfide technology, applied in non-active ingredients medical preparations, medical preparations containing active ingredients, wave energy or particle radiation treatment materials, etc., can solve the problem of uncontrollable release rate, poor water solubility, biological phase problems such as small capacitance, to achieve the effect of improving the poor solubility, good dispersibility and uniform size of the donor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of Conjugated Polymer P1
[0038]
[0039] 1) Weigh 12.31g of the compound and 0.18g of tetrabutylammonium bromide into a single-necked flask with a stirrer, add 4mL of 1,6-dibromohexane, and then add 40mL of 50wt% potassium hydroxide solution. Stirring under the conditions for 30 min, cooling to room temperature, adding 1M HCl to adjust the solution to be neutral, then adding water and dichloromethane to extract three times, keeping the organic phase, and purifying by column chromatography to obtain compound 2.
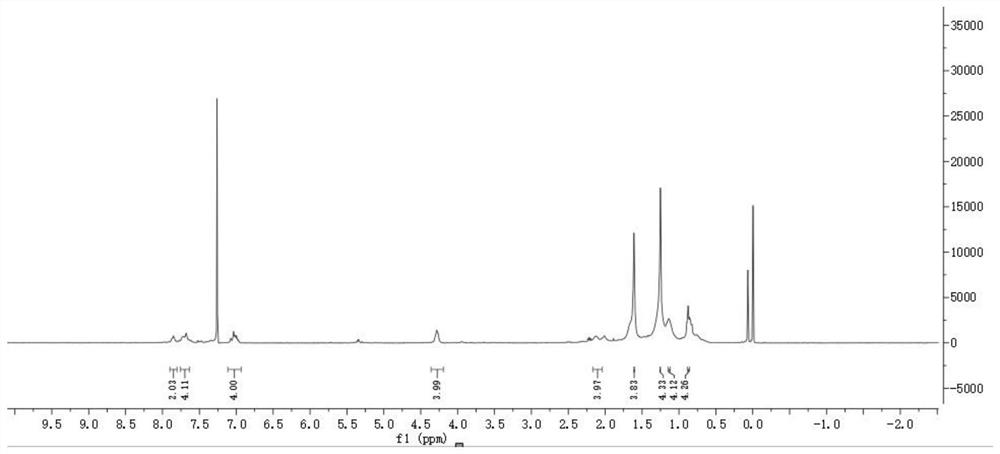
[0040] 1 H NMR (400MHz, CDCl 3 )δ (ppm): 7.53 (d, J = 8.0Hz, 2H), 7.48-7.44 (m, 4H), 3.30 (t, J = 6.8Hz, 4H), 1.95-1.91 (m, 4H), 1.71- 1.64(m,4H),1.23-1.17(m,4H), 1.13-1.05(m,4H),0.63-0.55(m,4H).
[0041] 2) Weigh compound 21.02g, potassium acetate 1.19g, bipinacol borate 1.32g, PdCl 2 (dppf) 0.038g was added to a 250mL eggplant-shaped bottle with a stirring bar, and 20mL of 1,4-dioxane was added, and stirred at 85°C for 24h under a...
Embodiment 2
[0048] Example 2: Preparation of water-soluble H2S donor P2
[0049]
[0050] Weigh 9.6mg of acrylamide, 137.8mg of N-isopropylacrylamide, 4.8mg of ADTOR, and 1.0mg of azobisisobutyronitrile, add them into a cylindrical solvent storage bottle with a magnet, seal the Schlenk tube with a high vacuum valve, and freeze -Aspiration-thawing, cycle three times, stir at 80°C for 12h, cool to room temperature, add 10mL of deionized water for dialysis, and obtain polymer P2.
Embodiment 3
[0051] Example 3: Preparation of azaboron fluoride complexed dipyrromethene dyes B1 (X=H)
[0052]
[0053] 1) Weigh 1.10g of compound 6 in a flask, add 10mL of ethanol and stir to dissolve, then add 1.03g of compound 5, add 4mL of 10% NaOH aqueous solution dropwise into the flask under rapid stirring conditions, stir and react at 30°C for 9h, and react After the end, suction filtration, the filter cake was washed with water first, then washed with a mixed solution of ethanol and water, and dried to obtain compound 7;
[0054] 1H NMR (400MHz, CDCl 3 )δ (ppm): 8.02 (d, J = 7.2Hz, 2H), 7.77 (d, J = 15.6 Hz, 1H), 7.58 (d, J = 8.4Hz, 2H), 7.43 (d, J = 15.6Hz ,1H),6.96-6.91(m,4H),4.08-3.97(m,4H),1.88-1.74(m,4H),1.49-1.25(m,12H),0.91(t,J=13.6Hz,6H ).
[0055] 2) Weigh 1.67g of compound 7 into a flask, add 10mL of methanol and stir to dissolve, add 1.25g of nitromethane and 749.7mg of diethylamine, and stir for 7 hours; after the reaction, neutralize the reaction solution with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com