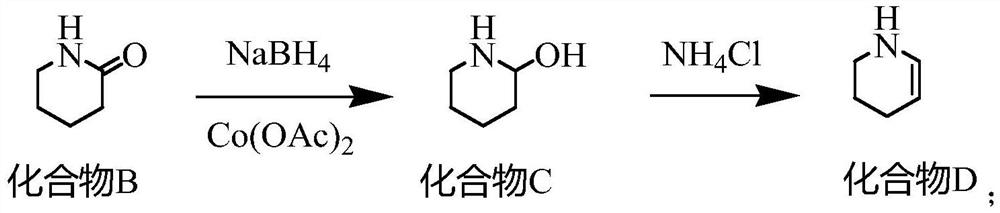
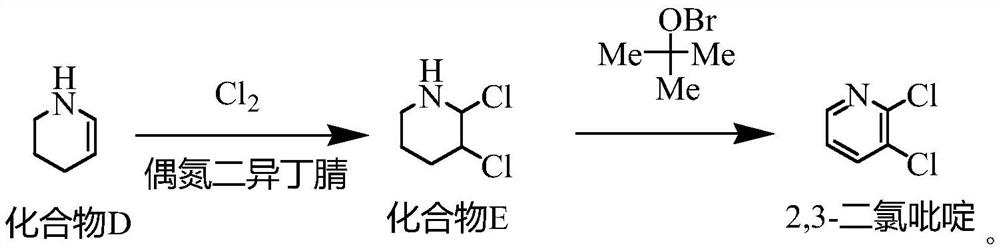
Synthesis method of 2, 3-dichloropyridine
A dichloropyridine and synthesis method technology, applied in the direction of organic chemistry, can solve the problems of high production cost, low synthesis yield, affecting the yield and purity of the final product, and achieve the effect of good quality and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The technical solution of this patent will be further described in detail below in conjunction with specific embodiments.
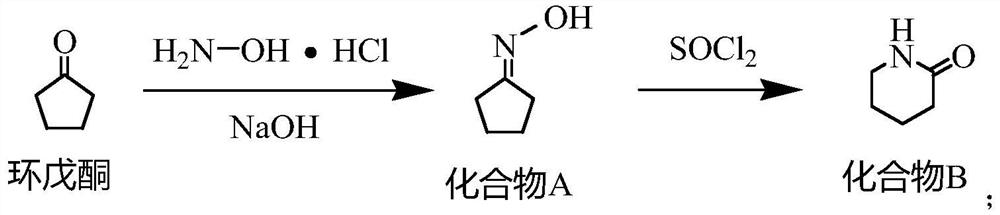
[0027] One, the synthetic method of compound B
[0028] Put water (504.72g) and sodium hydroxide (60g, 1.5mol) into a 1000mL four-necked bottle, stir to dissolve, then add hydroxylamine hydrochloride (104.24g, 1.5mol) in batches, after the addition is complete, control the temperature at 20-30°C, add dropwise Cyclopentanone (84.12g, 1.0mol) was added dropwise for about 1 hour. After the drop was completed, the temperature was kept for 2.5 hours. The reaction mixture was extracted by adding dichloromethane, and the dichloromethane layer was taken for GC analysis. The residual cyclopentanone was less than 0.5%. . Add dichloromethane (504.72g), stir for 20min, let stand for 1h, separate the dichloromethane layer MC①, add dichloromethane (504.72g) for the water layer, stir for 20min, let stand for 1h, separate the dichloromethane layer MC②, Combine M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com