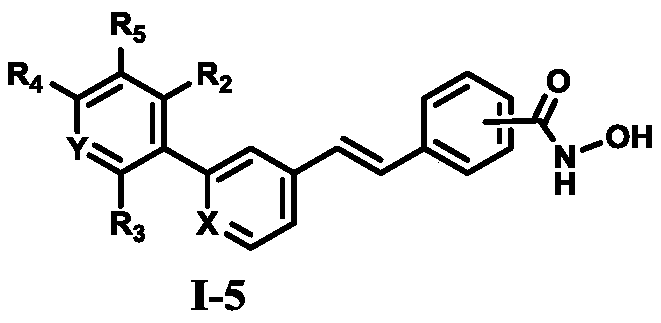
Hydroxamic acid group-containing diarylethene LSD1/HDACs double-target inhibitors as well as preparation method and application thereof
A technology of diarylethene and hydroxamic acid is applied in the field of application of diarylethene LSD1/HDACs dual-target inhibitors to prepare antitumor drugs, and achieves good selectivity, is conducive to popularization and application, and has good in vitro performance. The effect of antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 (E)-4-(2-(2-bromo-pyridin-4-yl) alkenyl) synthetic of methyl benzoate (I-3a)
[0023]
[0024] Add compound I-1a (376mg, 2.0mmol) and I-2a (601.1mg, 2.1mmo) to a 50ml two-necked flask, dissolve with 10ml of anhydrous DMF, add t-BuOK (673.3mg, 6.0mmol) under ice-cooling After the addition was completed, the reaction was stirred at room temperature for 0.5 hours, and then the reaction system was slowly added to ice water (50 mL), and a solid was precipitated. The solid was collected by suction filtration, separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 396.4 mg of compound I-3a, a white solid, yield: 62.3%, Mp: 106-107°C. 1 H NMR (400 MHz, CDCl 3 )δ8.36(d,1H,J=5.2Hz),8.08(d,2H,J=8.4Hz),7.60-7.57(m,3H),7.34-7.29(m,2H),7.04(d,1H ,J=16.4Hz),3.95(s,3H). 13 CNMR (101MHz, CDCl 3 )δ166.57, 150.44, 146.96, 143.04, 139.95, 133.46, 130.39, 130.20, 127.05, 126.87, 125.16, 120.09, 52.27. HRMS (ESI) calcd ...
Embodiment 2
[0025] Synthesis of embodiment 2 (E)-4-(2-(2-bromo-pyridin-4-yl) alkenyl) methyl benzoate (I-3b)
[0026]
[0027] According to the method of Example 1, compound I-2b (601.1mg, 2.1mmol) was used to replace I-2a to obtain 288.3 mg of target compound I-3b, a white solid, yield: 45.4%, Mp: 73-74°C. 1 H NMR (400MHz, CDCl 3 )δ8.33(d, 1H, J=5.2Hz), 8.21(t, 1H, J=2.0Hz), 8.00(dt, 1H, J 1 =1.2Hz,J2 =8.0Hz),7.70(dt,1H,J 1 =1.6Hz,J 2 =8.0Hz),7.57(s,1H),7.48(t,1H,J=8.0Hz),7.35-7.30(m,2H),7.03(d,1H,J=16.4Hz),3.96(s,3H ).HRMS(ESI)calcd for C 15 h 12 BrNNaO 2 [M+Na] + :339.9944,Found:339.9943.
Embodiment 3
[0028] The synthesis of embodiment 3 (E)-3-(3-bromostyryl) methyl formate (I-3c)
[0029]
[0030] According to the method of Example 1, compound I-1b (370 mg, 2.0 mmol) was used to replace I-1a to obtain 480 mg of target compound I-3c, yield: 75.6%, Mp: 177-178 ° C. 1 H NMR (400MHz, DMSO-d 6 ) δ7.97(d, 2H, J=8.4Hz), 7.89(t, 1H, J=2.0Hz), 7.74(d, 2H, J=8.4Hz), 7.64(dt, 1H, J 1 =1.2Hz,J 2 =8.0Hz),7.51-7.48(m,1H),7.43(d,2H,J=4.0Hz),7.37(t,1H,J=8.0Hz),3.86(s,3H). 13 C NMR (101MHz, DMSO-d 6 )δ166.41, 141.90, 139.66, 131.31, 131.20, 130.09, 129.65, 129.38, 129.04, 127.28, 126.38, 122.76, 52.57. HRMS (ESI) calcd for C 16 h 13 BrNaO 2 [M+Na] + :338.9991,Found:338.9996.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com