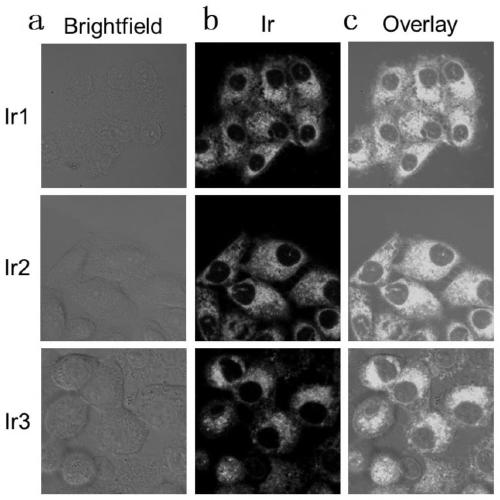
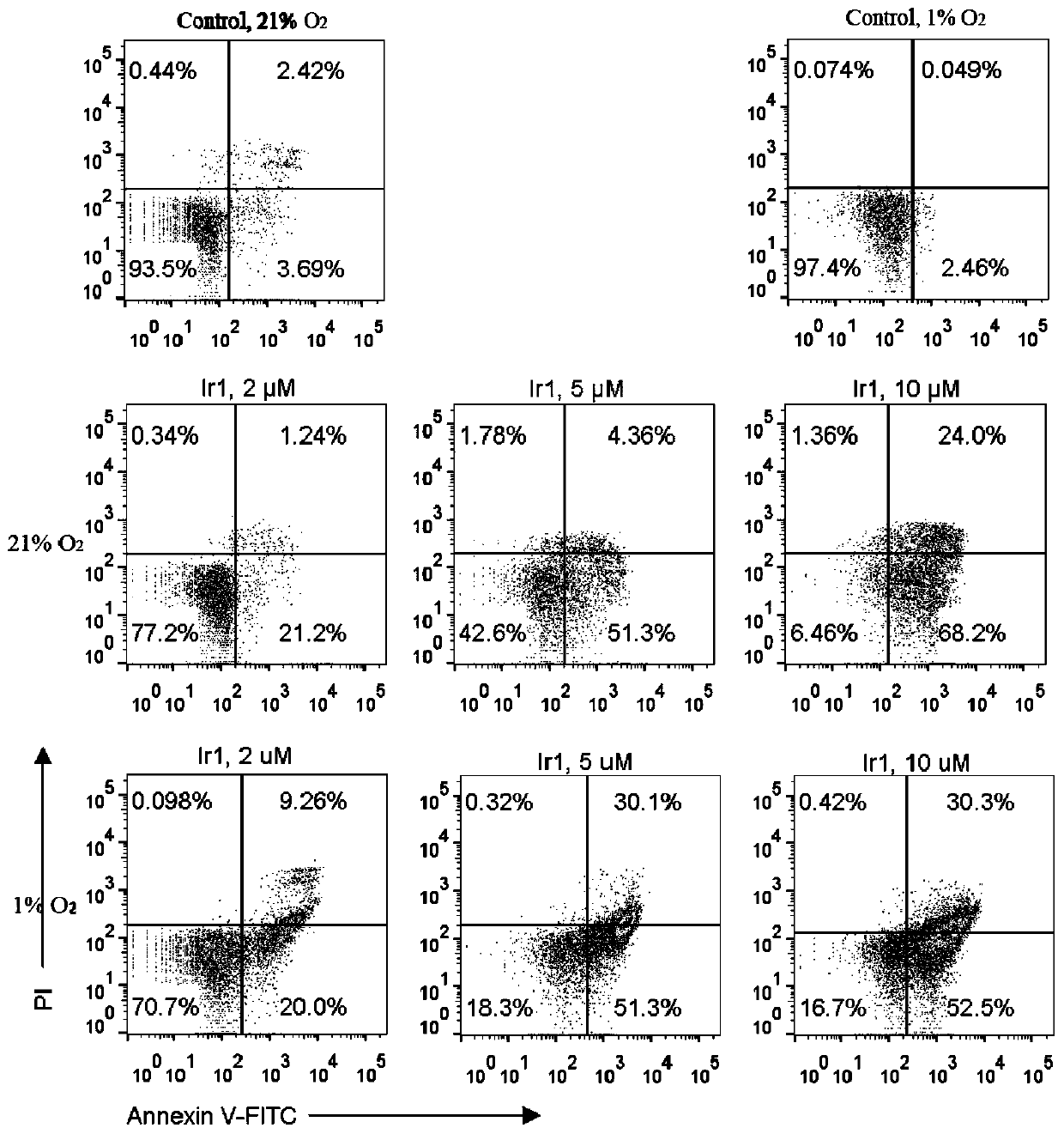
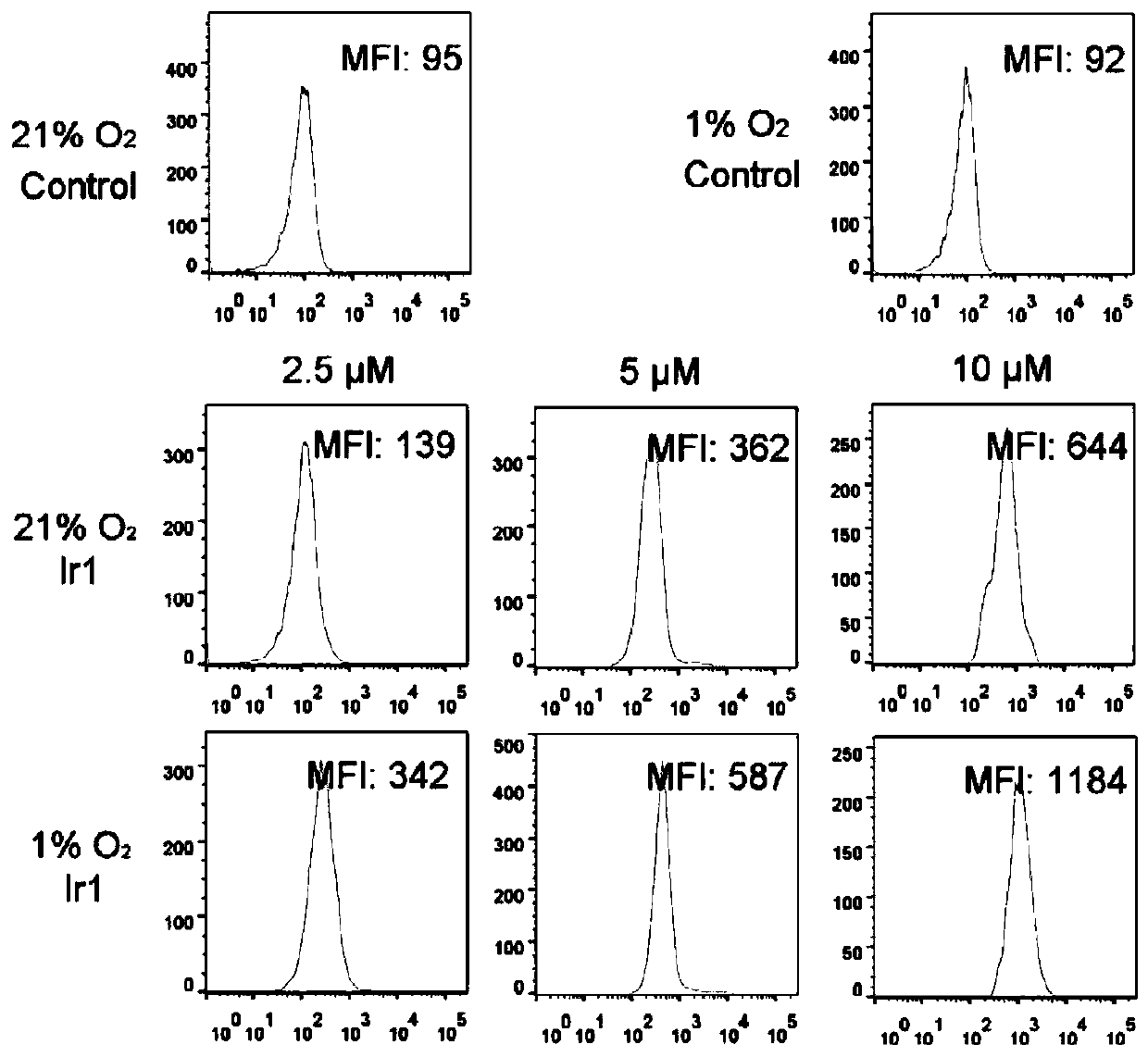
Cyclometalated iridium metformin complex and preparation method and application thereof
A technology of metformin and complexes, which is applied in the field of cyclometalated iridium metformin complexes and their preparation, can solve problems such as anti-tumor activity not mentioned, and achieve excellent hypoxic anti-tumor activity, good inhibitory activity, and enhanced cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 ring metal iridium metformin complex Ir1
[0055] The preparation method of ring metal iridium metformin complex Ir1 comprises the following steps:
[0056]S1. After mixing iridium trichloride hydrate and ligand at a molar ratio of 1:2.2, dissolve it in a mixed solvent of ethylene glycol ether and ultrapure water (volume ratio: 3:1), and stir at 135°C under reflux The coordination reaction was carried out for 24 hours, cooled to room temperature and suction filtered, and the obtained solid product was washed three times with ultrapure water and diethyl ether, and dried in vacuum to obtain an iridium precursor;
[0057] S2. After mixing the iridium precursor (121.4mg, 0.1mmol) obtained in step S1 with metformin (66.2mg, 0.4mmol), dissolve in dichloromethane / methanol mixed solvent (volume ratio is 1.5:1), in N 2 Potassium tert-butoxide (89.8mg, 0.8mmol) was added under protection, and the reaction was carried out at 35°C for 25h. After the...
Embodiment 2
[0063] The preparation of embodiment 2 ring metal iridium metformin complex Ir2
[0064] The preparation method of ring metal iridium metformin complex Ir2 comprises the following steps:
[0065] S1. After mixing iridium trichloride hydrate and ligand at a molar ratio of 1:2.5, dissolve it in a mixed solvent of ethylene glycol ether and ultrapure water (volume ratio: 2:1), and stir at 130°C under reflux Carry out the coordination reaction for 22 hours, cool to room temperature and suction filter, the obtained solid product is washed 3 times with ultrapure water and diethyl ether, and vacuum-dried to obtain the iridium precursor;
[0066] S2. After mixing the iridium precursor (121.4mg, 0.1mmol) obtained in step S1 with metformin (66.2mg, 0.4mmol), dissolve in dichloromethane / methanol mixed solvent (volume ratio is 2:1), in N 2 Potassium tert-butoxide (89.8mg, 0.8mmol) was added under protection, and the reaction was carried out at 40°C for 30h. After the reaction was complete...
Embodiment 3
[0072] The preparation of embodiment 3 ring metal iridium metformin complex Ir3
[0073] The preparation method of ring metal iridium metformin complex Ir3 comprises the following steps:
[0074] S1. After mixing iridium trichloride hydrate and ligand at a molar ratio of 1:3, dissolve it in a mixed solvent of ethylene glycol ether and ultrapure water (volume ratio: 4:1), and stir at 140°C under reflux Carry out the coordination reaction for 26 hours, cool to room temperature and suction filter, the obtained solid product is washed 3 times with ultrapure water and diethyl ether, and vacuum-dried to obtain the iridium precursor;
[0075] S2. After mixing the iridium precursor (121.4mg, 0.1mmol) obtained in step S1 with metformin (66.2mg, 0.4mmol), dissolve in dichloromethane / methanol mixed solvent (volume ratio is 1:1), in N 2 Potassium tert-butoxide (89.8mg, 0.8mmol) was added under protection, and the reaction was carried out at 30°C for 20h. After the reaction was completed,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com