Erythrocyte preserving fluid and application thereof
A technology for red blood cells and preservation solution, applied in the field of cell preservation solution, can solve the problems of loss of phospholipids, reduction of red blood cell viability, failure of preservation period to meet biological experiments and clinical needs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation and washing of embodiment 1 erythrocytes
[0032] After the leukocytes were removed from 3 servings of fresh A-type healthy human suspension red blood cells purchased from Tianjin Blood Center, an appropriate amount of red blood cells was taken for mixing. Add 10 times the volume of sodium chloride injection, pipette to mix, place in a centrifuge and centrifuge at 3000rr for 5min, take out the centrifuged type A red blood cell test tube, suck off the supernatant, and repeat this process twice. After the third centrifugation, the supernatant was sucked off, and the remaining packed red blood cells were kept for future use. Type B packed red blood cells were prepared in a similar manner.
Embodiment 2
[0033] The preparation of embodiment 2 erythrocyte preservation solution
[0034] Prepare 6 groups of red blood cell preservation solutions according to the following ingredients.
[0035] (1) GMA preservation solution: adenine 1.8mmol / L; glucose 111mmol / L; mannitol 79.6mmol / L; sodium citrate 14mmol / L; MgCl 2 1.02mmol / L; Sodium chloride 23.32mmol / L; KH 2 PO 4 1.9mmol / L;Na 2 HPO 4 1.0mmol / L.
[0036] (2) Experimental group 1: adenine 1.8mmol / L, glucose 111mmol / L, mannitol 79.6mmol / L, sodium citrate 14mmol / L, MgCl 1.02mmol / L, sodium chloride 23.32mmol / L, KH 2 PO 41.9mmol / L, Na 2 HPO 4 1.0mmol / L, chloramphenicol 77.4mmol / L, neomycin sulfate 140.3mmol / L, EGCG 1μmol / L.
[0037] (3) Experimental group 2: the concentration of EGCG was changed to 5 μmol / L, and the concentration of other components was the same as that of experimental group 1.
[0038] (4) Experimental group 3: the concentration of EGCG was changed to 10 μmol / L, and the concentration of other components w...
Embodiment 3
[0041] The preparation of embodiment 3 erythrocyte suspension
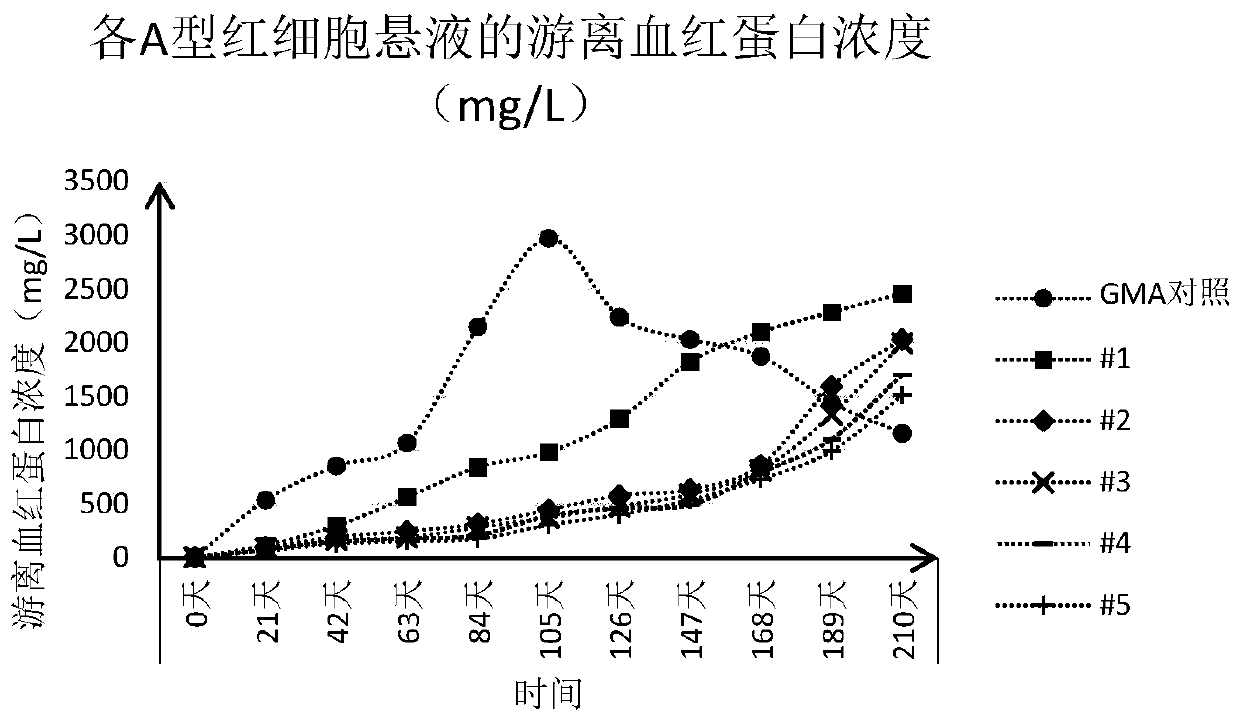
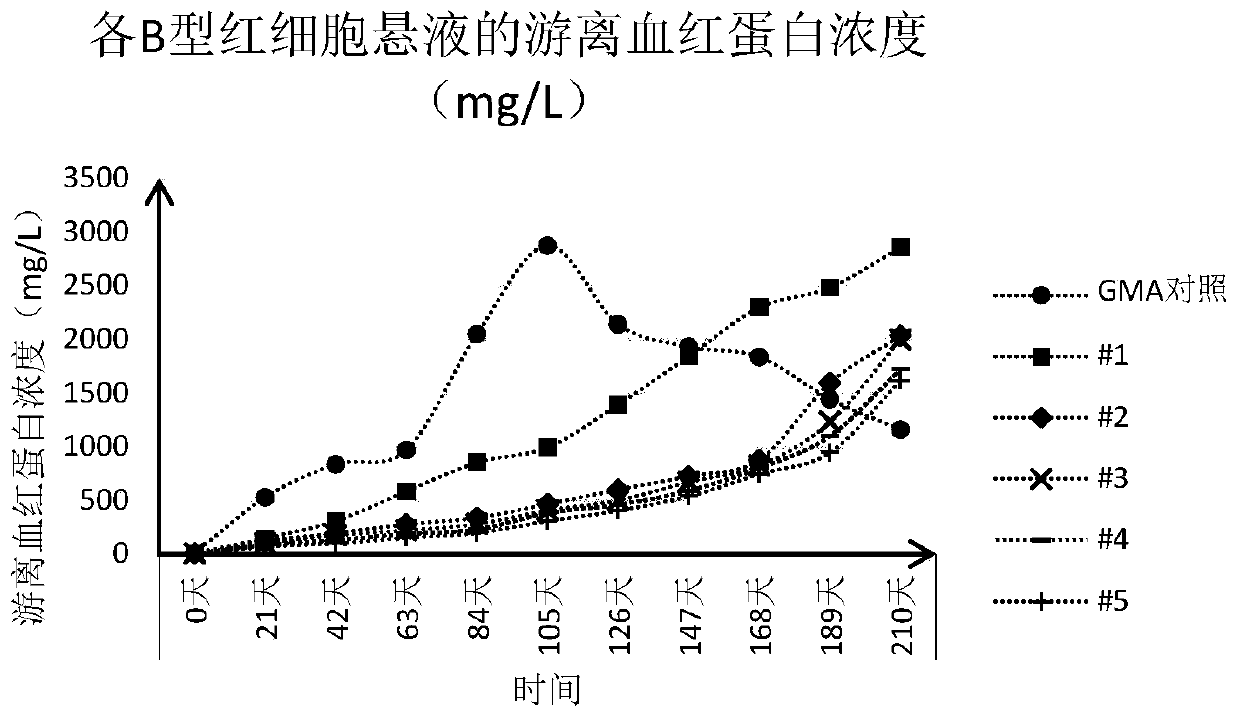
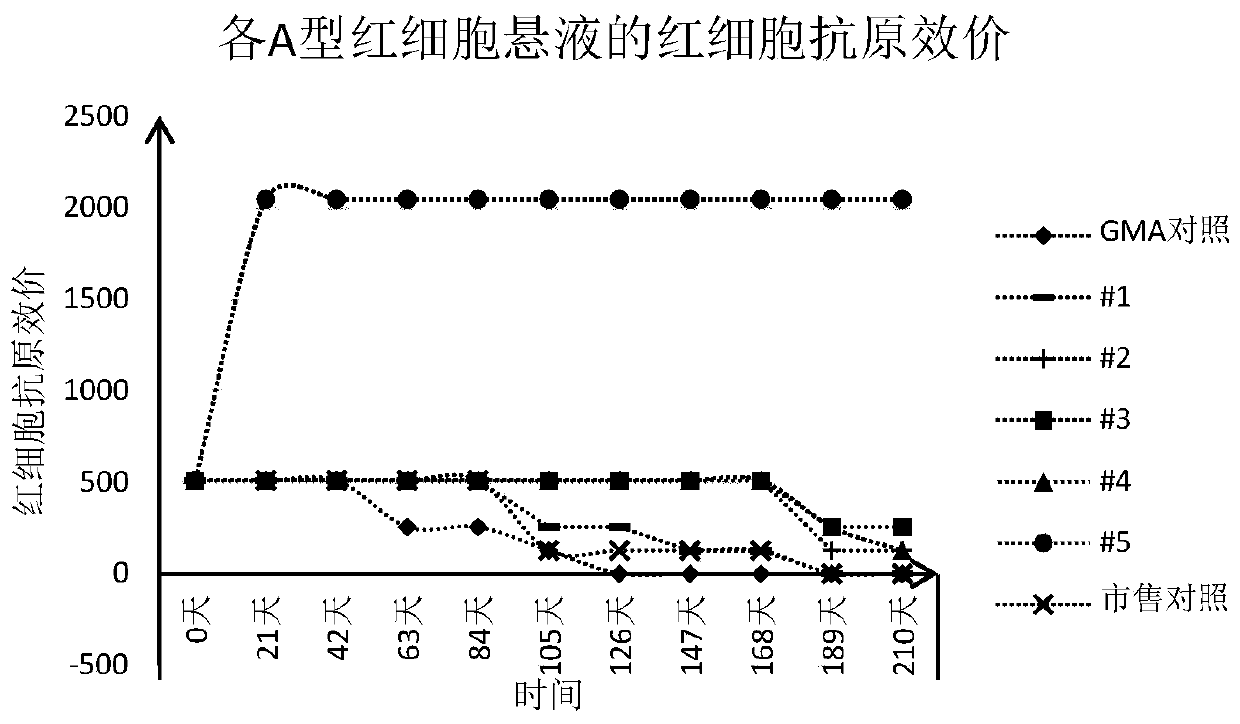
[0042] According to the "Clinical Blood Transfusion Testing Operation Procedures", suck out 100 μL of the packed red blood cells prepared in Example 1 and add them into the dropper bottle filled with 10 mL of the red blood cell preservation solution prepared in Example 2 to obtain 0.8 % concentration of red blood cell suspension. For each red blood cell preservation solution, two bottles of red blood cell suspension were prepared, one bottle was used for detection, and the other bottle was used for static observation. Store in refrigerator at 2-8°C.
[0043] For the convenience of illustration, the erythrocyte suspension preserved in the GMA preservation solution is also referred to as "GMA control" in this paper, and the erythrocyte suspension preserved in the experimental group 1-5 preservation solution in Example 2 is correspondingly recorded as "#1" to "#5".
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com