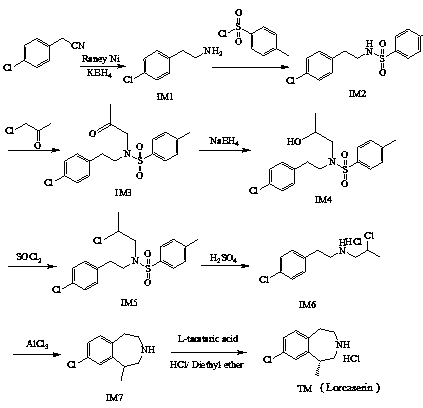
Method for preparing lorcaserin
A technology for lorcaserin and chlorophenyl, which is applied in the field of preparing lorcaserin, can solve the problems of complicated operation, many steps and high production cost, and achieves the effects of good reaction selectivity, high product purity and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of p-chlorophenethylamine (IM1)
[0028] In a 250mL three-necked flask, add 15.16g (0.10mol) p-chlorophenylacetonitrile, add 180 mL cocatalyst ethanol, then add 5.87g (0.10mol) Raney Ni, 16.18g (0.30mol) KBH 4 , stirred at 25°C, the reaction was complete after 2 hours, stopped stirring, filtered with suction, and removed ethanol by rotary evaporation, added 200 mL of ethyl acetate, 200 mL of water, and adjusted the pH to 1 with 6 mol / L hydrochloric acid, separated the ethyl acetate layer, and water Add 200mL ethyl acetate to the layer, adjust the pH to 13 with 40% NaOH, separate the ethyl acetate layer, extract the water layer with 200mL ethyl acetate, combine the ethyl acetate layers, add anhydrous sodium sulfate to dry, filter with suction, spin Ethyl acetate was distilled off to obtain 14.32 g of light yellow oily liquid with a yield of 92.0%.
[0029] IM1 Characterization Data:
[0030] 1 H NMR (500 MHz, CDCl 3 ) δ 7.23 (d, J = 8.1 Hz, 2H), 7.09 (d, ...
Embodiment 2
[0032] Preparation of p-chlorophenethylamine (IM1)
[0033] In a 250mL three-necked flask, add 15.13g (0.10mol) of p-chlorophenylacetonitrile, add 150 mL of cocatalyst methanol, then add 5.88g (0.10mol) of Raney Ni, 11.36g (0.30mol) of NaBH 4 , stirred and reacted at 30°C for 3 hours, and the post-reaction treatment was the same as in Example 1, wherein the acid pH was adjusted to 2, and the base pH was adjusted to 12 after extraction and removal of impurities to obtain 13.23 g of light yellow oily liquid with a yield of 85.0%.
Embodiment 3
[0035] Preparation of N-(2-(4-chlorophenyl)ethyl))-4-methylbenzenesulfonamide (IM2)
[0036]In a 250mL three-necked flask, add 15.56g (0.10mol) of p-chlorophenethylamine, add 175mL of dichloromethane to dissolve it, add 14mL of triethylamine (0.10mol) under stirring at 25°C, and then add 19.07g in an ice bath (0.10mol) p-toluenesulfonyl chloride, stirred for 30 minutes and then moved to room temperature. After 4 hours, the reaction was complete and filtered with suction. After the filtrate was spin-dried, 50mL of ethanol was added and heated to 78°C for recrystallization to obtain 26.35g of IM2 white solid. 85.1%, HPLC purity 99.9%.
[0037] IM2 Characterization Data:
[0038] ESI-MS: m / z = 310[M+H] +
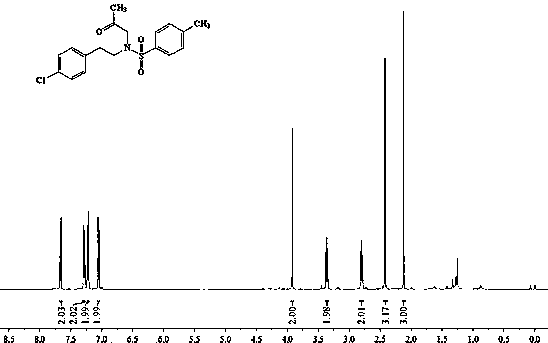
[0039] 1 H NMR (500 MHz, CDCl 3 ) δ 7.69 (d, J = 8.3 Hz, 2H), 7.28 (d, 2H), 7.21(d, 2H), 7.02 (d, J = 8.4 Hz, 2H), 4.79 (s, 1H), 3.18 (t , J = 7.0 Hz, 2H), 2.74 (t, J = 7.0 Hz, 2H), 2.44 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com