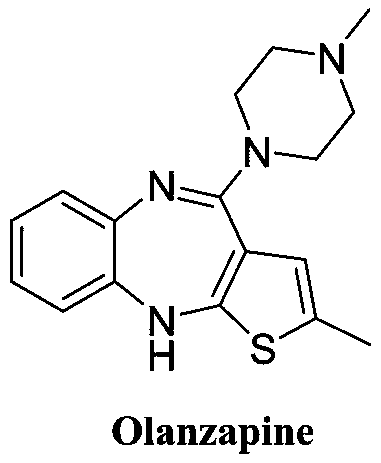
Synthesis method of olanzapine related substances like compound I and compound II
A technology related to substances and synthesis methods, applied in the direction of organic chemistry, etc., can solve the problems of no preparation methods for compounds I and II, no synthesis of compounds, etc., and achieve the effects of significant technical and economic benefits, reliable material guarantee, and short route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Synthetic method of the present invention comprises the following steps:
[0033] S1. Synthesis of compound I: add olanzapine, organic solvent and water into the reaction flask, add Oxone and sodium hydroxide in sequence, and continue the reaction until the olanzapine spots disappear, and then adjust the pH of the system to 6-7, After extraction and recrystallization, compound I was obtained;
[0034] S2: Synthesis of Compound II: Dissolve Compound I in an organic solvent, add inorganic lye at 0°C, then add acetyl chloride, continue to stir the reaction at 0°C until Compound I disappears, separate and extract, pass through a silica gel column Purification by chromatography affords compound II.
[0035] In some preferred embodiments, in the step S1, the organic solvent is selected from one or more of methanol, ethanol, dichloromethane, chloroform, N,N-dimethylformamide or N-methylpyrrolidone Mixed solvents. Most preferred is N,N-dimethylformamide.
[0036] In some pr...
Embodiment 1
[0051] Embodiment 1 of the present invention provides a kind of preparation method of compound I, and its synthetic route is as follows:
[0052]
[0053] Specifically adopt the following method to prepare:
[0054]Add olanzapine (8.0g, 25.6mmol), N,N-dimethylformamide (40mL) and water (80mL) into the reaction flask, add Oxone (7.9g, 25.6mmol) under stirring, continue to Stir at 25°C for 2h, then add solid sodium hydroxide (3.1g, 76.8mmol), continue stirring at 25°C for 1h, TLC detects that the starting material disappears. Use 2M hydrochloric acid to adjust the pH of the system to 6-7, add dichloromethane (120 mL) to the reaction liquid, separate and extract, and wash the organic layer with water (50 mL×3) and saturated brine (50 mL×3) successively. The organic layer was dried with anhydrous sodium sulfate, filtered with suction, and the filtrate was distilled off under reduced pressure. The crude product was recrystallized from acetonitrile (55 mL) to obtain the pure com...
Embodiment 2
[0061] Embodiment 2 of the present invention provides a kind of preparation method of compound I:
[0062] Add olanzapine (6.0g, 19.2mmol), N-methylpyrrolidone (30mL) and water (60mL) into the reaction flask, and add under stirring (5.9g, 19.2mmol), continue to stir at 25°C for 2h after the addition, then add solid sodium hydroxide (2.3g, 57.6mmol), continue to stir at 25°C for 1h, TLC detects that the starting material disappears. Use 2M hydrochloric acid to adjust the pH of the system to 6-7, add dichloromethane (90 mL) to the reaction solution, separate and extract, and wash the organic layer with water (40 mL×3) and saturated brine (40 mL×3) successively. The organic layer was dried with anhydrous sodium sulfate, filtered with suction, and the filtrate was evaporated under reduced pressure. The crude product was recrystallized from acetonitrile (41 mL) to obtain pure compound I.
[0063] Using this method, 4.6 g of a light yellow solid was prepared, with a yield of 79.2%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com