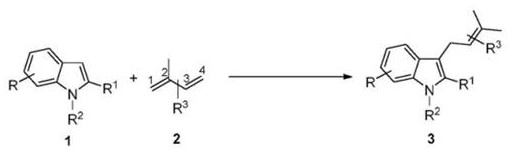
A method for introducing isopentenyl at the C3 position of indole
A technology of isopentenyl and isoprene, which is applied in organic chemistry, bulk chemical production, etc., can solve the problems of poor atom economy and increased reaction steps, and achieve the effect of low price and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention will be described below with specific examples, but the protection scope of the present invention is not limited to these examples.
[0028] 1. Pd-catalyzed reaction of indole and isoprene
[0029] In a 2.0 mL sealed pressure-resistant reaction tube, add Pd catalyst (5 mol% of indole), phosphine ligand (5 mol% of indole), additives (30 mol% of indole), and indole 1a (0.2 mol% of indole) in turn. mmol, 23.4 mg), dissolved in 0.2 mL of solvent, then added isoprene 2a (3.0 equiv (0.6 mmol), 60 μL), reacted at 70 ° C for 24 h, and added mes-trimethoxybenzene as an internal standard after completion, GC- The yield of target product 3a was detected by FID.
[0030]
[0031] Table 1. Effects of catalysts, ligands, additives, and solvents on the reaction
[0032]
[0033]
[0034] It can be seen from the results in Table 1 that when the molar ratio of indole 1a and isoprene 2a is 1:3, the reaction is carried out at 70 °C, using THF as solvent, P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com