Detoxifying pharmaceutical composition and use thereof
A kind of composition and medicine technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
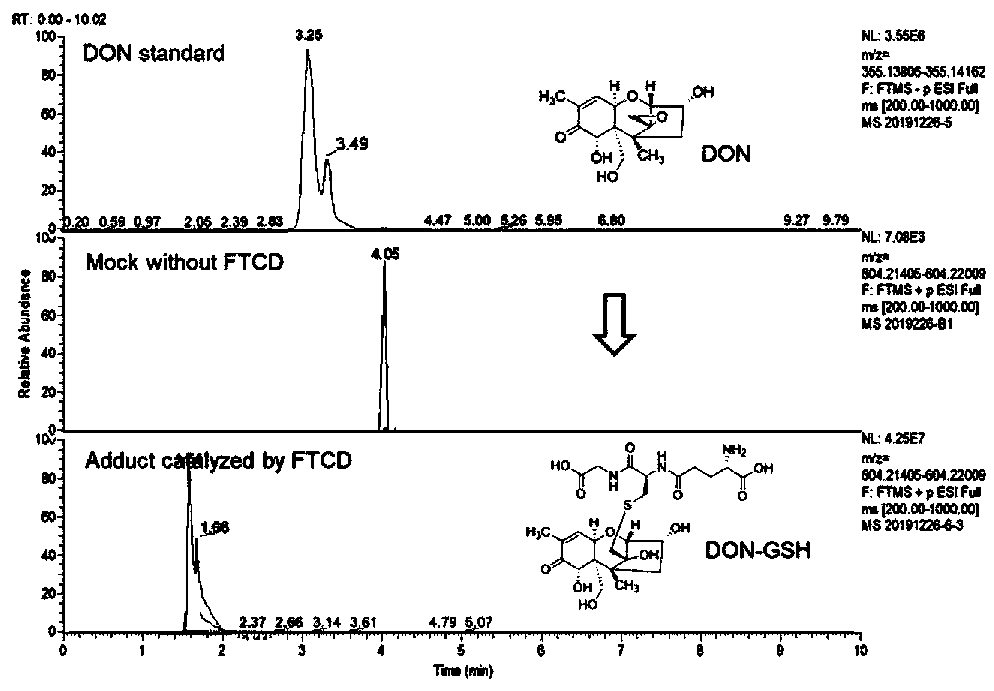
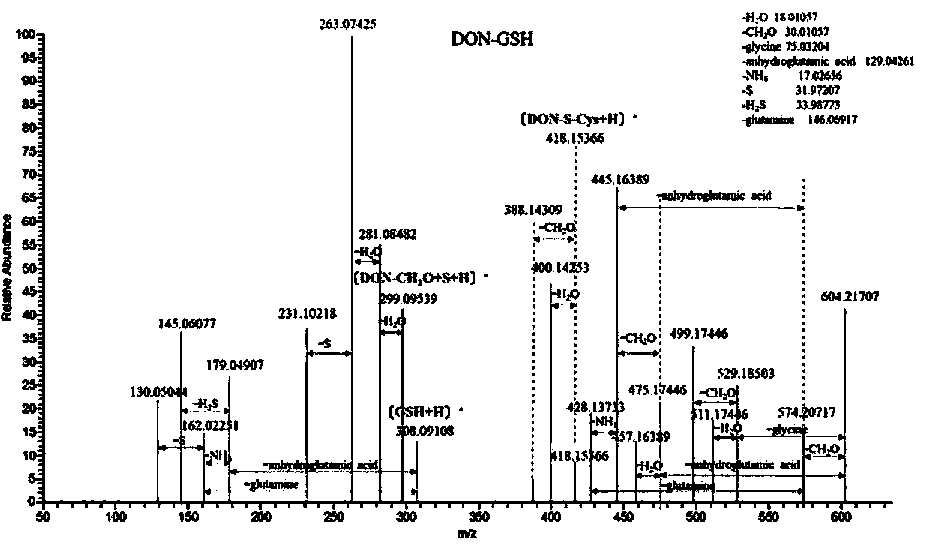
[0059] 1. The active polypeptide FTCD catalyzes the removal of epoxy groups from trichothecenes
[0060] 1. Experimental method:
[0061] 1.1 In vitro enzymatic reaction:
[0062] 0.05mol / L phosphate buffer solution (PBS): 0.05mol / L Na 2 HPO 4 Mix with 0.05mol / L KH2PO4 at a ratio of 3:2, pH=7.0; 0.1mol / L glutathione (GSH): weigh 3.073g of glutathione, add distilled water to 100ml; 0.5mg / ml Trichothecenes (DON, 3DON, 15ADON, FUS-X, NIV, T2, H-T2, DAS): 1 mg of trichothecenes, add distilled water to 2 ml, filter to sterilize.
[0063] Dissolve DON, 3-DON, 15-ADON, NIV, DAS, HT-2, T-2 toxins (1 mg) in freshly prepared GSH (30.7 mg, 100 μmol) in PBS buffer, and add active peptides, Insulate in a 20°C water bath for 24h.
[0064] 1.2 LC-HRMS ( / MS) analysis
[0065] After the in vitro reaction solution was filtered through a 0.22 μm filter membrane, it was transferred to an injection vial for LC-HRMS detection. Using Thermo Scientific TM Q Exactive TM Combined quadrupole Or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com