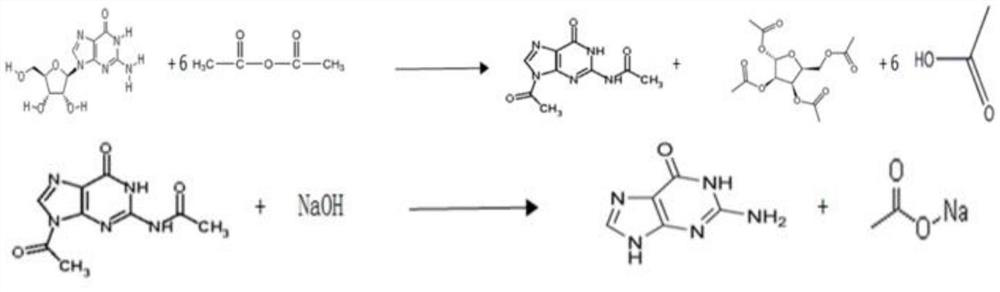
A kind of method utilizing guanosine to synthesize guanine
A technology of guanine and diacetylguanine, applied in chemical instruments and methods, organic chemistry, sugar derivatives, etc., can solve the problems of complex extraction process, poor product quality, long time consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Mix acetic anhydride and guanosine, which account for half of the total amount, to obtain a mixed solution 1; mix the remaining acetic anhydride and boric acid evenly, and heat up to 121°C to obtain a mixed solution 2; mix the mixed solution 1 with 5 cubic meters Add slowly to the mixed solution 2 at a rate of 1 hour, wherein the mass ratio of guanosine, mixed solution 1 and mixed solution 2 to the total amount of acetic anhydride and boric acid is 1:6.6:0.008; Insulate and react for 6 hours; then distill part of the acetic acid under the condition of a slight negative pressure below 0.04MPA, the amount of distilled acetic acid is 25% of the total amount of acetic acid produced in theory, after the distillation is completed, keep warm at 121°C and continue the reaction for 6.5 hours; after the reaction is completed, cool down to 4°C, and then filtered or suction filtered to collect the filter cake and filtrate; the filter cake was further washed 3 times with acetic a...
Embodiment 2
[0042] (1) Mix acetic anhydride and guanosine, which account for half of the total amount, to obtain a mixed solution 1; mix the remaining acetic anhydride and boric acid evenly, and heat up to 120°C to obtain a mixed solution 2; mix the mixed solution 1 with 4 cubic meters Add slowly to the mixed solution 2 at a rate of 1 hour, wherein the mass ratio of guanosine, mixed solution 1 and mixed solution 2 to the total amount of acetic anhydride and boric acid is 1:6.6:0.008; Insulate and react for 6.5 hours; then distill part of the acetic acid under the condition of a slight negative pressure below 0.04MPA, the amount of distilled acetic acid is 20% of the total amount of acetic acid produced in theory; after the distillation is completed, keep warm at 120°C and continue the reaction for 7 hours; after the reaction is completed, cool down to 0°C, and then filtered or suction filtered to collect the filter cake and filtrate; the filter cake was further washed 4 times with acetic a...
Embodiment 3
[0047] (1) Mix acetic anhydride and guanosine, which account for half of the total amount, to obtain mixed solution 1; mix the remaining acetic anhydride and boric acid evenly, and heat up to 125°C to obtain mixed solution 2; mix mixed solution 1 with 6 cubic meters Add slowly to the mixed solution 2 at a rate of 1 hour, wherein the mass ratio of guanosine, mixed solution 1 and mixed solution 2 to the total amount of acetic anhydride and boric acid is 1:6.6:0.008; Insulate and react for 6 hours; then distill part of the acetic acid under the condition of a slight negative pressure below 0.04MPA, and the amount of acetic acid evaporated is 30% of the total amount of acetic acid produced in theory; 5°C, then filter or suction filter to collect the filter cake and filtrate; the filter cake is further washed twice with acetic anhydride and dried in vacuum to obtain diacetylguanine;
[0048] (2) The filtrate obtained in step (1) is concentrated under reduced pressure or distilled t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com