Preparation of mitochondria targeting near-infrared fluorescent probe with aggregation-induced emission effect
A technology of aggregation-induced luminescence and fluorescent probes, applied in the field of preparation of mitochondria-targeted near-infrared fluorescent probes, can solve problems such as poor photostability, unfavorable long-distance tracking, and strong photobleaching, and achieve low background interference and good Photostability and photobleaching resistance, the effect of great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of intermediate mCy-Br
[0026] The synthesis method is attached figure 1 shown.
[0027] Specific steps: Take a dry 10 mL two-necked bottle equipped with a magnet, add IR-780 (50 mg, 0.0775 mmol) and 5-bromocatechol (27.0 mg, 0.143 mmol), replace with vacuum-nitrogen three times, Under the condition of nitrogen protection, 2ml of anhydrous DMF and 0.1ml of anhydrous triethylamine were added to the reaction flask, heated to 80°C for 4h, and vacuum-dried to obtain a crude product. The crude product was separated and purified by flash chromatography (dichloromethane:methanol = 20 / 1, v / v) to obtain a blue solid compound (yield about 62.9%). Characterized by NMR and mass spectrometry. 1H NMR (400 MHz, 298 K, CDCl3): δ 8.53 (d, 1H),7.46−7.25 (m, 6H), 7.15(s, 1H), 6.26 (d, 1H), 4.22 (t, 2H), 2.73 (t, 2H),2.67 (t, 2H), 1.96−1.91 (m, 4H), 1.25 (s, 6H), 1.09 (t, 3H). 13C NMR (400MHz, 298 K, CD3OD): δ 177.3 , 162.6, 154.5, 141.6, 144.0, 133.4, 128.9, 127.1,126.6, 1...
Embodiment 2
[0029] Probe Synthesis
[0030] The synthesis method is attached figure 1 shown.
[0031] Specific steps: Add intermediate 1 (30 mg, 0.048 mmol), 4-(1,2,2-tristyryl)-phenylboronic acid pinacol ester (40 mg, 0.087 mmol), K2CO3 (0.338 g, 2.45 mmol), vacuum-nitrogen replacement 3 times, added under nitrogen protection, added 4ml THF, Pd(PPh3)4 (5 mg, 0.004 mmol), 1ml H2O, heated to reflux, added dichloromethane to extract after 24 hours 4 times, the organic phases were combined and concentrated to obtain the crude product. The crude product was separated and purified by flash chromatography (dichloromethane:methanol = 20 / 1, v / v) to obtain a blue solid compound (yield about 24%). Characterized by NMR and mass spectrometry. 1H NMR (400 MHz, 298 K, CDCl3): δ8.58 (d, 1H), 7.52 (s, 1H), 7.37(d, 2H), 7.26 (s, 1H), 7.16−7.06 (m,22H) , 6.96(s, 1H), 6.08 (d, 1H), 4.06 (t, 2H), 2.60-2.63 (t, 2H), 1.89−1.91 (m, 4H), 1.29 (s, 6H), 1.07 (t , 3H). 13C NMR (400 MHz, 298 K, CDCL3): Δ 175.7...
Embodiment 3
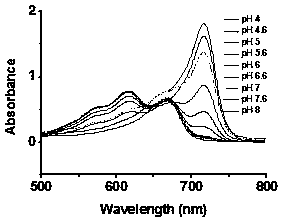
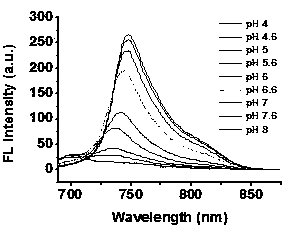
[0033] Prepare probe 10μM 70% PBS / THF solutions with different pH values, measure on a visible-ultraviolet spectrophotometer, the results are as attached figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com