Novel coronavirus advantage epitope fusion protein, diagnosis reagent and application
A dominant epitope and fusion protein technology, which is applied in the field of diagnostic reagents and novel coronavirus dominant epitope fusion proteins, can solve the problems of low sensitivity of missed detection, difficult inter-batch control, complex production process, etc., and achieve biological stability Good, improved biocompatibility, good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of novel coronavirus 2019-nCoV multi-dominant epitope fusion recombinant protein:
[0046] The dominant epitopes of the new coronavirus 2019-nCoV N protein, S protein, and E protein were screened by computational prediction, and linked together by flexible polypeptides (GGGS) to form a 2019-nCoV virus multi-dominant epitope fusion protein. Entrusted to Beijing Deaoping Biotechnology Co., Ltd. for production and preparation, the purity is greater than 90%.
[0047] In the examples of the present invention, 8 kinds of recombinant proteins were constructed, of which 4 kinds adopted the fusion protein scheme described in the technical solution of the present invention, and the other 4 kinds adopted the traditional protein construction according to the retrieval sequence, and the basic sequences were consistent with the market recombinant proteins.
[0048] fusion sequence 1
[0049] N proteins 348-406, S proteins 452-611, E proteins 30-75, linker GGGS underline...
Embodiment 2
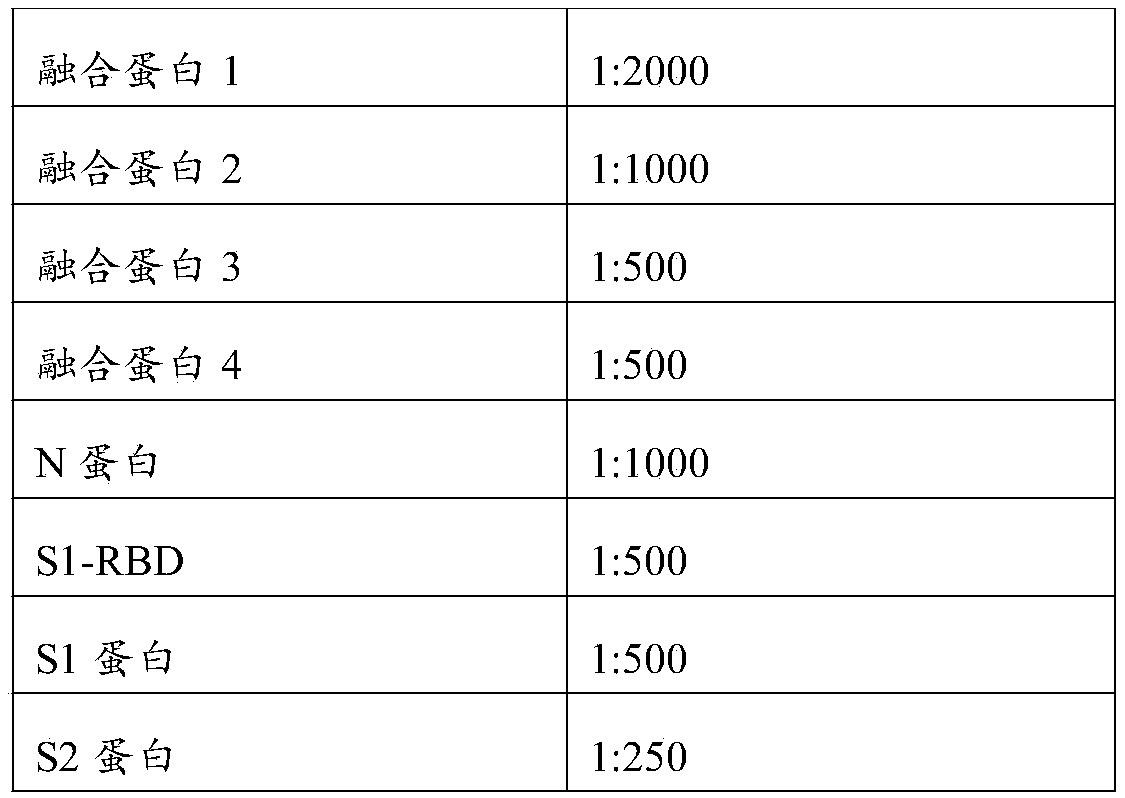
[0078] Performance verification of 8 recombinant protein raw materials
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com