Terminal alkenyl-containing negative liquid crystal compound and synthesis method thereof
A technology of negative liquid crystal and synthesis method, which is applied in the field of negative liquid crystal compound and its synthesis, can solve the problem that the liquid crystal display cannot well satisfy the low voltage driving fast response, poor low temperature stability, low clearing point and low optical anisotropy, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Step (1), in a 500mL three-necked flask, add 12.1g (50mmol) 2,2', 3,3'-tetrafluoro[1,1'-biphenyl]-4-phenol, 6.7g (55mmol) 1- Chloro-4-methoxybutane and 13.8g (100mmol) of potassium carbonate were fully dissolved in 50mL of N,N-dimethylformamide, heated to an external temperature of 90°C, and reacted for 5 hours;
[0121] Cool, add 150mL water and 100mL ethyl acetate to separate the liquid, extract the aqueous phase with 2×100mL ethyl acetate, combine the organic phases, wash with 3×80mL water, dry, and spin dry;
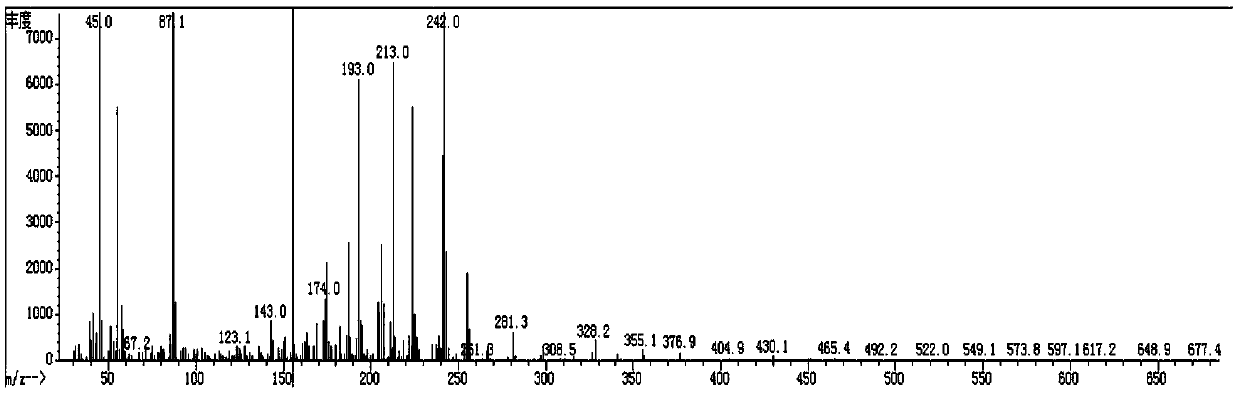
[0122] Spread 20 g of 200 mesh silica gel, pass the organic phase through the column, and evaporate the solvent to dryness at 50° C. to obtain 18 g of red liquid. Add 40mL of ethanol to recrystallize at -30°C for 3h, filter with Buchner funnel to obtain 12.5g of white solid (compound 3-1: 2,2',3,3'-tetrafluoro-4-(4-methoxybutoxy base)-1,1'-biphenyl), purity 99.1%, yield 76.2%; its mass spectrum is as figure 1 shown; 1 HNMR (δ, ppm, CDCl 3 ,500MHz):1.48(m, ...
Embodiment 2
[0138] Step (1), in a 500mL three-necked flask, add 12.1g (50mmol) 2,2', 3,3'-tetrafluoro[1,1'-biphenyl]-4-phenol, 6.7g (55mmol) 1- Chloro-4-methoxybutane and 13.8g (100mmol) of potassium carbonate were fully dissolved in 50mL of isopropanol, heated to an external temperature of 90°C, and reacted for 5 hours;
[0139] Cool, add 150mL water and 100mL ethyl acetate to separate the liquid, extract the aqueous phase with 2×100mL ethyl acetate, combine the organic phases, wash with 3×80mL water, dry, and spin dry;
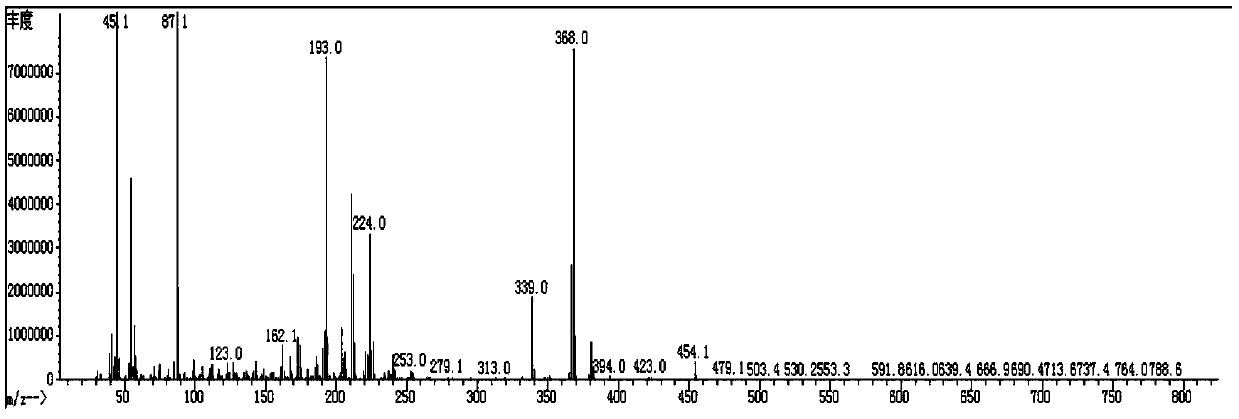
[0140] Spread 20g of 200 mesh silica gel, pass the organic phase through the column, and evaporate the solvent to dryness at 50°C to obtain 15g of red liquid. Add 40 mL of ethanol for recrystallization and freeze at -30°C for 3 h, and suction filter through a Buchner funnel to obtain 12.1 g of a white solid (compound 3-1: 2,2',3,3'-tetrafluoro-4-(4-methoxybutoxy base)-1,1'-biphenyl), purity 98.8%, yield 74.1%; its mass spectrum and main 1 H-NMR is the same as compound...
Embodiment 3
[0156] Step (1), in a 500ml three-necked flask, 24.2g (100mmol) 2,2', 3,3'-tetrafluoro[1,1'-biphenyl]-4-phenol, 12.3g (100mmol) 1- Chloro-4-methoxybutane and 24g (170mmol) of potassium carbonate were fully dissolved in 100mL of N,N-dimethylformamide, heated to an external temperature of 120°C, and reacted for 2 hours;
[0157] Cool, add 180mL water and 120mL ethyl acetate to separate the liquid, extract the aqueous phase with 2×100mL ethyl acetate, combine the organic phases, wash with 3×80mL water, dry, and spin dry;
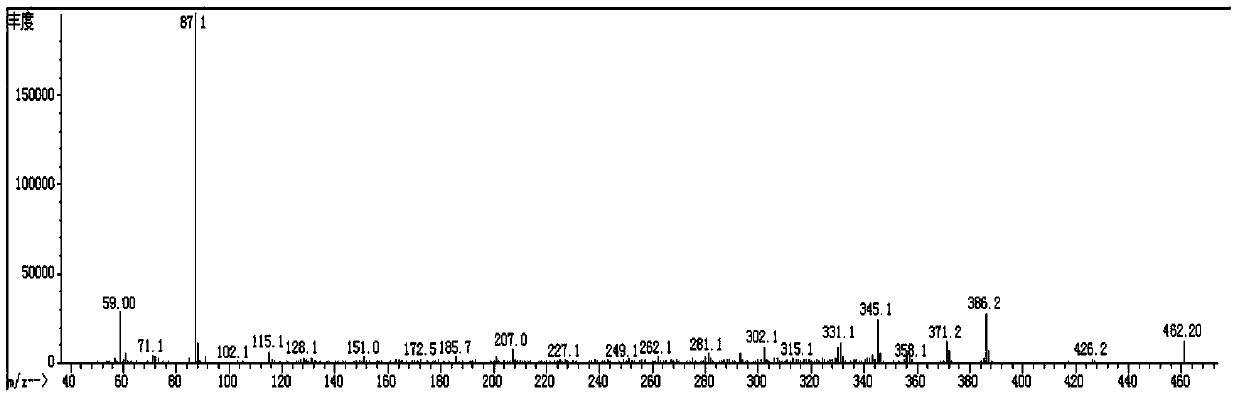
[0158] Spread 20g of 200 mesh silica gel, pass the organic phase through the column, evaporate the solvent to dryness at 50°C to obtain 36g of red liquid, add 40mL of ethanol to recrystallize at -30°C for 3 hours, and filter with Buchner funnel to obtain 24.7g of white solid (Compound 3-1: 2 ,2',3,3'-tetrafluoro-4-(4-methoxybutoxy)-1,1'-biphenyl), purity 98.5%, yield 75.3%; its mass spectrum and main 1 H-NMR is the same as compound 3-1 of Example 1.
[0159] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com