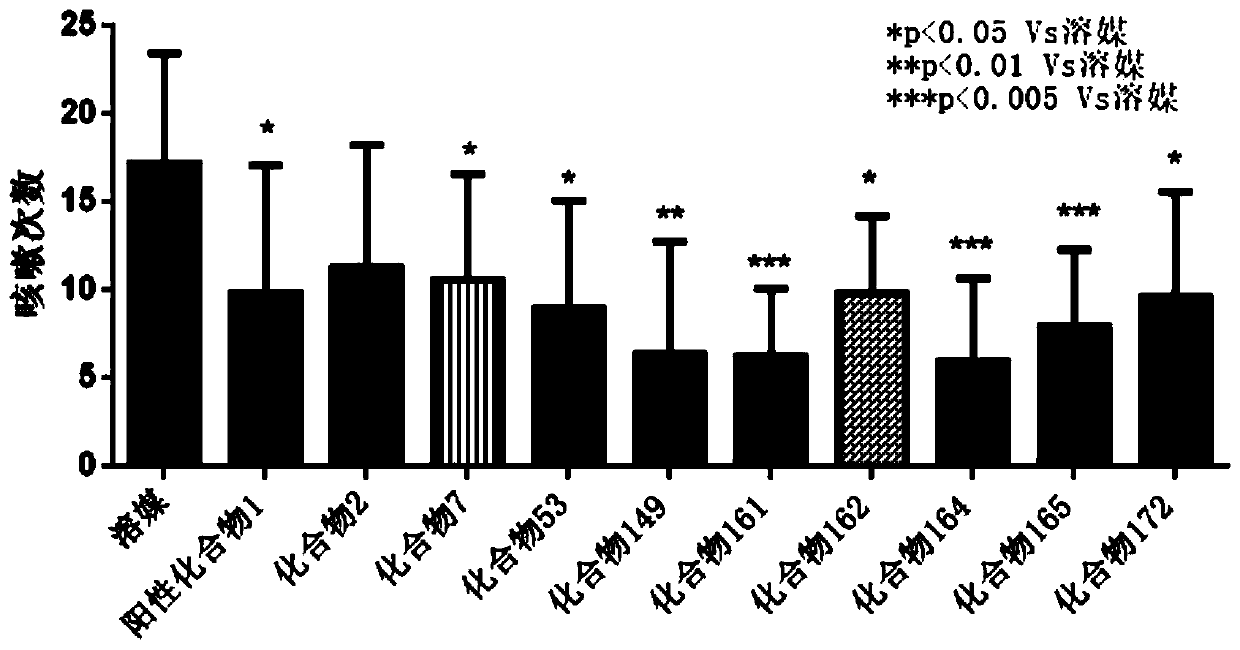
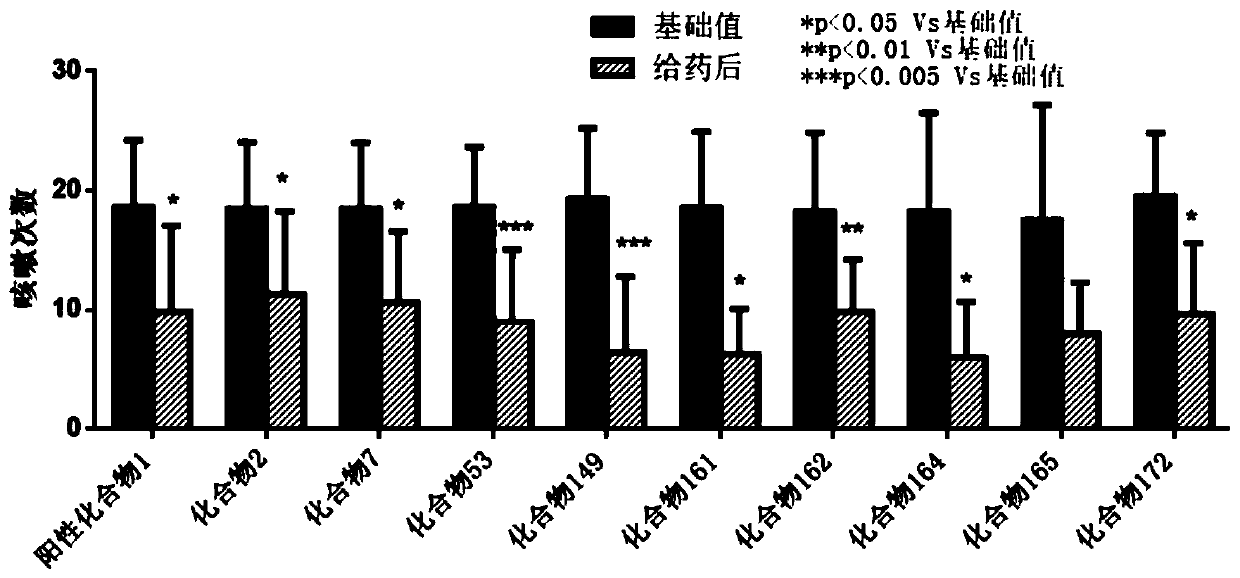
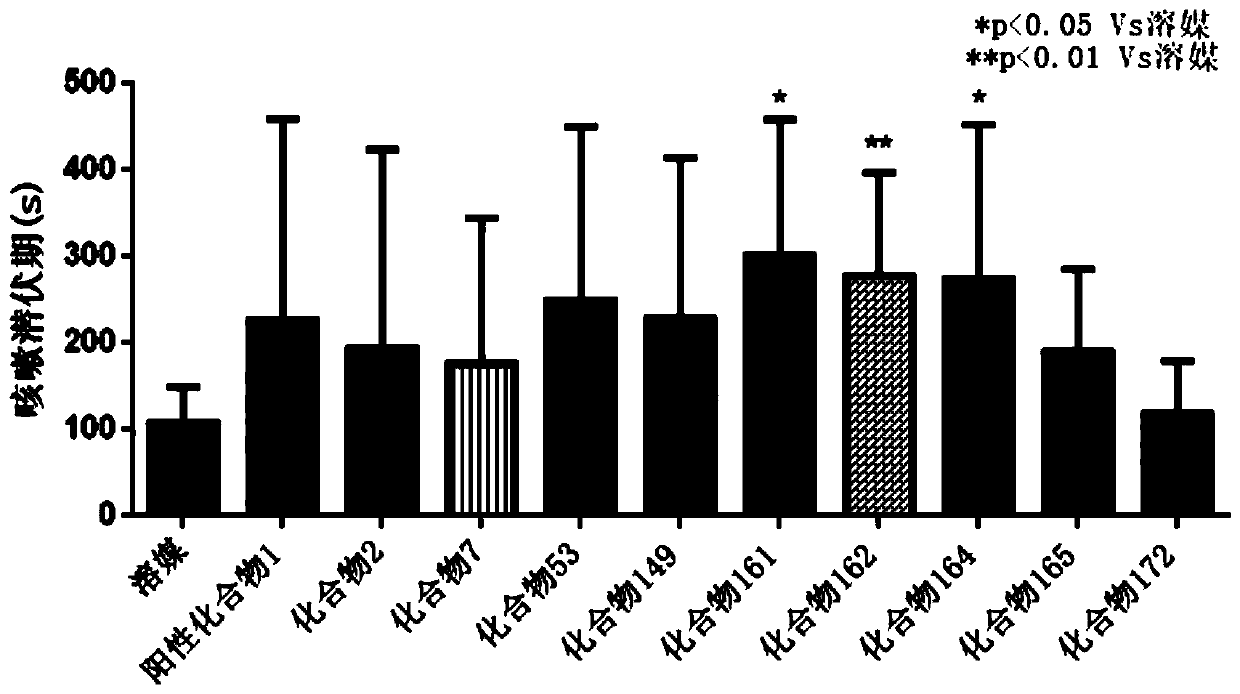
Heterocyclic compound, intermediate, preparation method and application thereof
A technology of heterocyclic compounds, applied in the field of heterocyclic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0558]
[0559] Step (1) Preparation of (R)-2-formyl-morpholine-4-formic acid tert-butyl ester
[0560]
[0561] Compound 1-1 (10g, 460mmol), TEMPO (0.073g, 0.44mmol), sodium bromide aqueous solution (0.5M, 10mL, 41mmol) and dichloromethane (100mL) were cooled to a temperature of 0°C-5°C, Sodium bicarbonate (2.3g, 23mmol) was added to the sodium hypochlorite (1.5M, 34ml, 58mmol) solution to adjust the pH of the solution to 9.3, and the solution was slowly dropped into the reaction system over 30 minutes, and the stirring was continued for half an hour after the addition was completed , heated to 20° C. and added water (50 mL), and added dichloromethane to extract the aqueous phase. After multiple extractions, the organic phase was combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure, and column chromatography obtained compound 1-2. Orange-yellow oily liquid (6g, 60%). LC-MS: [M+H] + = 216.4.
[0562] Step (2) Preparation of (...
Embodiment 2
[0591]
[0592] Step (1) Preparation of 2,6-difluoro-4-bromobenzaldehyde
[0593]
[0594] Get 3,5-difluorobromobenzene (10g, 51.8mmol) and dissolve in anhydrous THF (80mL), N 2 Under protection, add LDA (31mL, 62.5mmol) dropwise at -78°C, continue stirring for 1h after the dropwise addition is complete, add DMF (4mL, 51.9mmol), stir for 30min, then add the reaction solution into NH 4 Cl solution, and extracted with ethyl acetate, the organic phase was washed with NaCl, dried over anhydrous magnesium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the concentrated crude product was obtained by column chromatography to obtain compound 2-2, a yellow solid (8g, yield 70% ).
[0595] Step (2) (S)-2-((2-(2,6-difluoro-4-bromophenyl)-7-methylimidazo[1,2-a]pyridin-3-yl)-methanol base) the preparation of morpholine-4-formic acid tert-butyl ester
[0596]
[0597] Take compound 2-2 (200mg, 0.9mmol), 2-amino-4-picoline (100mg, 0.9mmol), compound 1-4...
Embodiment 3
[0609]
[0610] Step (1) (S)-2-((2-(2,6-difluoro-4-aminophenyl)-7-methylimidazo[1,2-a]pyridin-3-yl)-methanol base) the preparation of morpholine-4-formic acid methyl ester
[0611]
[0612] At room temperature, hydrochloric acid (1.5 mL) was added to a solution of compound 2 (145 mg, 0.32 mmol) in ethanol (3 mL), and the reaction solution was reacted for 1 h at 100 ° C. After the reaction was complete, it was cooled to room temperature and washed with 1 N NaHCO 3 The solution was quenched, extracted with DCM (5 mL×3), washed with saturated sodium chloride solution (5 mL×2), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a yellow oily liquid (90 mg). LC-MS: [M+H] + = 417.3.
[0613] Step (2) (S)-2-((2-(2,6-difluoro-4-(methoxycarbonylamino)phenyl)-7-methylimidazo[1,2-a]pyridine- Preparation of 3-yl)-methyl)morpholine-4-carboxylic acid methyl ester
[0614]
[0615] At 0°C, to a solution of Intermediate 3-1 (70mg, 0.17mmol) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com