Application of diosmetin in preparation of medicament for treating inflammatory bowel disease
A technology of inflammatory bowel disease and diosmin, which is applied in the direction of drug combinations, pharmaceutical formulas, organic active ingredients, etc., can solve the problems of inflammatory bowel disease and the unreported effect of intestinal flora, and achieve the alleviation or improvement of ulcerative Enteritis disease, good application prospect, no adverse effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
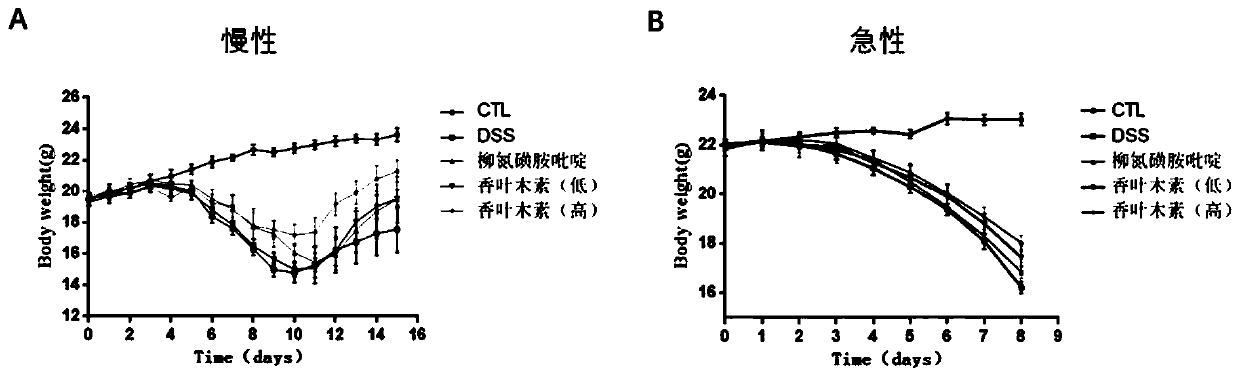
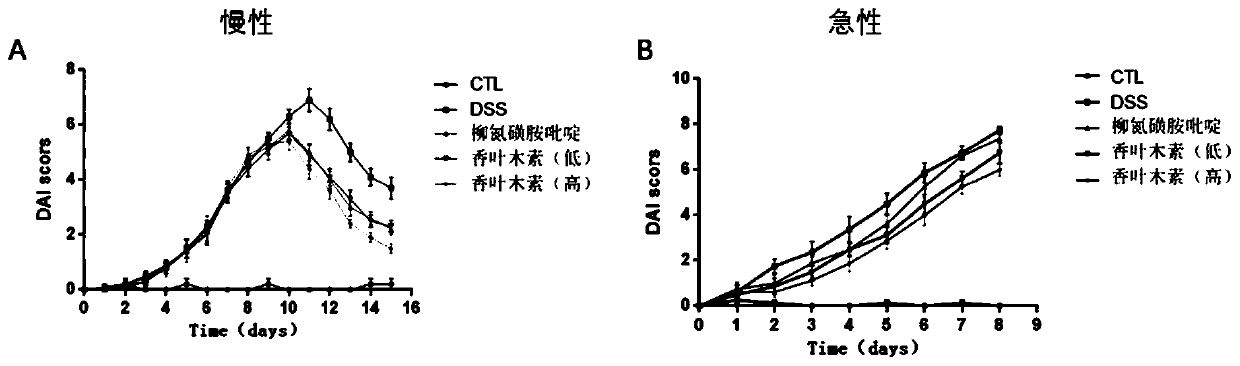
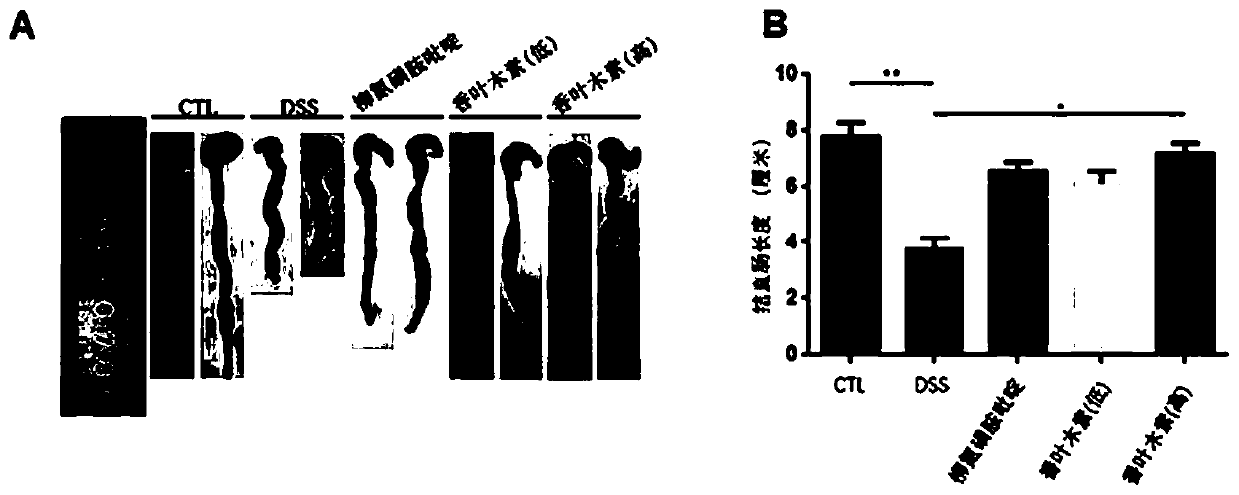
[0033] Effects of nintedanib on dextran sodium sulfate (DSS)-induced ulcerative colitis in mice
[0034] Chronic ulcerative colitis animal model preparation: 3% DSS was used to induce the chronic phase model of ulcerative colitis. During the adaptation period, the mice were allowed to eat and drink freely. After the initial adaptation period, the water was replaced with 3% (W / V ) of DSS (except the normal control group). The daily water intake of each mouse was calculated as 6 mL, and the DSS solution was supplemented to the daily water intake the next day, and the DSS solution was given for 7 days in total. After 7 days of administration, it was found that the mice had soft stools, diarrhea or bloody stools, and the mice were administered intragastrically. The mice in the normal control group and the model control group were given an equal volume of sodium carboxymethyl cellulose every day, and the mice in the positive control group Sulfasalazine 200 mg / kg was given daily, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com