Iliac vein stent
An iliac vein and proximal technology, applied in the field of medical devices, can solve problems such as inappropriateness and poor iliac vein compression treatment effect, and achieve the effects of reducing the influence of blood flow, reducing the risk of thrombosis, and reducing treatment costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
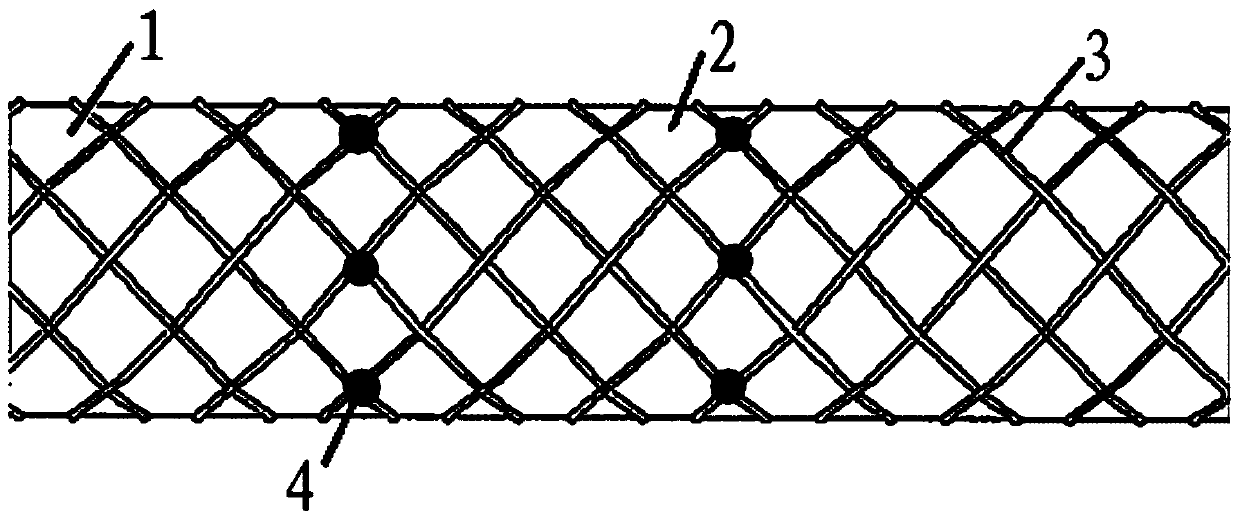
[0025] An iliac vein stent includes a proximal part, a middle part and a distal part connected in sequence, the proximal part and the distal part are degradable stents, and the middle part is a permanent stent.
[0026] The material of the permanent stent is nickel-titanium alloy. For example, the existing permanent stent approved for application in the iliac vein is the Ziver stent of Cook Company.
[0027] The degradable bracket is made of absorbable magnesium-based alloy. The proximal, middle and distal sections are joined by laser.
[0028] There are 4-6 developing places at the junction of the middle part, the proximal part and the distal part, and the developing places are tungsten developing points.
[0029] The length of the proximal portion is 18 mm, the length of the middle portion is 20 mm, and the length of the distal portion is 35 mm.
[0030] The rhomboid mesh support structure of the iliac vein stent.
Embodiment 2
[0032] An iliac vein stent includes a proximal part, a middle part and a distal part connected in sequence, the proximal part and the distal part are degradable stents, and the middle part is a permanent stent.
[0033] The material of the permanent support is platinum-chromium alloy.
[0034] The degradable scaffold is a polymer absorbable material. The proximal, intermediate and distal sections are connected by braiding.
[0035] There are 4-6 developing places at the junction of the middle part, the proximal part and the distal part, and the developing places are tungsten developing points.
[0036] The length of the proximal portion is 19 mm, the length of the middle portion is 21 mm, and the length of the distal portion is 40 mm.
[0037] The iliac vein stent has a rhombic mesh structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com