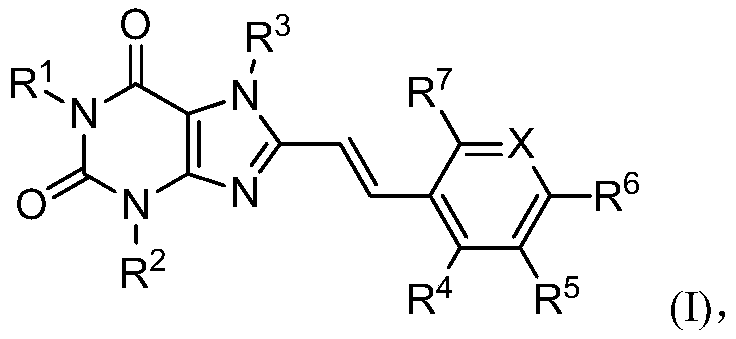
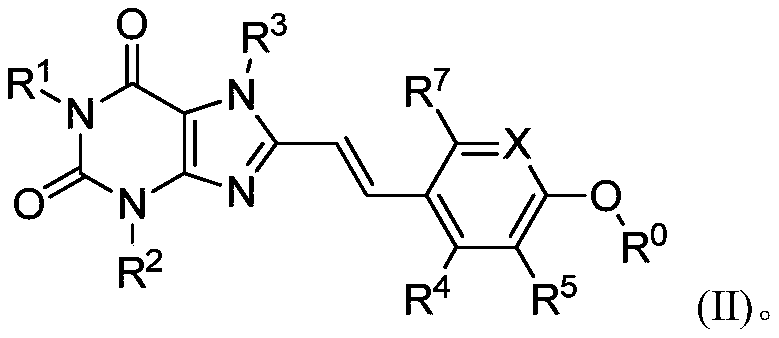
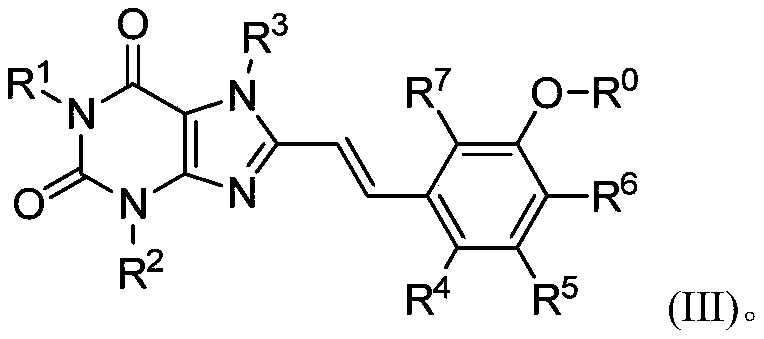
8-substituted aromatic ring vinyl xanthine derivative and application thereof
An aryl and heteroaryl technology, applied in the field of medicine, can solve problems such as the distribution limitation of adenosine A, and achieve the effects of good bioavailability, good clinical application prospects, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0276] Example 1 (E)-8-(4-((1,1-dioxotetrahydro-2H-thiopyran-4-yl)oxy)styryl)-1,3-diethyl Synthesis of -7-methyl-1H-purine-2,6(3H,7H)-dione
[0277]
[0278] Step 1) Synthesis of 1,1-dioxotetrahydro-2H-thiopyran-4-yl 4-methylbenzenesulfonate
[0279] At 25°C, p-toluenesulfonyl chloride (4.0g, 20.98mmol) and triethylamine (8.82mL, 63.0mmol) were added to a 100mL single-necked flask, dichloromethane (30mL) was added, and tetrahydro-2H- Thiopyran-4-ol 1,1-dioxide (4.1 g, 27.27 mmol), reacted for 24 hours. Add water (40mL), then add dichloromethane (20mL), separate the layers, collect the organic phase, spin dry under reduced pressure, separate and purify by column chromatography (petroleum ether / ethyl acetate (v / v)=10 / 1~5 / 1) The title compound was obtained as a pale yellow solid (4.2 g, 66%).
[0280] MS(ESI,pos.ion)m / z:305.1[M+H] + ;
[0281] 1 H NMR (400MHz, CDCl 3 )δ (ppm) 7.81 (d, J = 8.2Hz, 2H), 7.39 (d, J = 8.1Hz, 2H), 5.38–5.28 (m, 1H), 4.76–4.66 (m, 4H), 2.49...
Embodiment 2
[0300] Example 2 (E)-8-(4-((1,1-dioxotetrahydro-2H-thiopyran-4-yl)oxy)-3-fluorostyryl)-1,3 Synthesis of -diethyl-7-methyl-1H-purine-2,6(3H,7H)-dione
[0301]
[0302] Step 1) Synthesis of 4-((1,1-dioxotetrahydro-2H-thiopyran-4-yl)oxy)-3-fluorobenzaldehyde
[0303] The title compound of this step was prepared by referring to the method described in step 2 of Example 1, that is, 1,1-dioxotetrahydro-2H-thiopyran-4-yl 4-methylbenzenesulfonate (2.0g, 6.57mmol) and 3-fluoro-4-hydroxybenzaldehyde (1.38g, 9.86mmol) were prepared by reacting in N,N-dimethylformamide (10mL), and the crude product was separated and purified by silica gel column chromatography (petroleum ether / Ethyl acetate (v / v) = 10 / 1) gave the title compound as a light yellow solid (1.25 g, 70%).
[0304] MS(ESI,pos.ion)m / z:273.2[M+H] + ;
[0305] 1 H NMR (400MHz, CDCl 3 )δ (ppm) 9.90 (d, J = 1.9Hz, 1H), 7.67 (d, J = 8.8Hz, 2H), 7.15 (t, J = 7.8Hz, 1H), 4.81 (brs, 1H), 3.53– 3.34(m,2H),3.02–2.96(m,2H),2.60–2...
Embodiment 3
[0319] Example 3 (E)-8-(4-((1,1-dioxotetrahydro-2H-thiopyran-4-yl)oxy)-3-chlorostyryl)-1,3 Synthesis of -diethyl-7-methyl-1H-purine-2,6(3H,7H)-dione
[0320]
[0321] Step 1) Synthesis of 4-((1,1-dioxotetrahydro-2H-thiopyran-4-yl)oxy)-3-chlorobenzaldehyde
[0322] The title compound of this step was prepared by referring to the method described in step 2 of Example 1, that is, 1,1-dioxotetrahydro-2H-thiopyran-4-yl 4-methylbenzenesulfonate (0.5g, 3.28mmol) and 3-chloro-4-hydroxybenzaldehyde (468mg, 3.0mmol) were prepared by reacting in N,N-dimethylformamide (10mL), and the crude product was separated and purified by silica gel column chromatography (petroleum ether / acetic acid Ethyl ester (v / v)=10 / 1) afforded the title compound as a yellow solid (0.605 g, 70%).
[0323] MS(ESI,pos.ion)m / z:289.2[M+H] + .
[0324] Step 2) (E)-3-(4-((1,1-dioxotetrahydro-2H-thiopyran-4-yl)oxy)-3-chlorophenyl)propene acid synthesis
[0325] The title compound of this step was prepared ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com