Preparation method of lipoic acid process impurity
A technology for processing impurities and lipoic acid, applied in the direction of organic chemistry, etc., to achieve the effect of single product, high selectivity, and avoiding the formation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
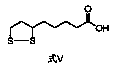
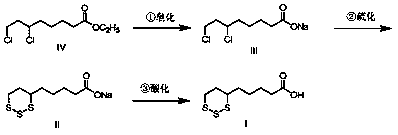
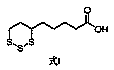
[0020] Put 10.00g (41.5mmol) of ethyl 6,8-dichlorooctanoate, (100mL) water, and 1.83g (45.7mmol) of sodium hydroxide into a 500mL three-necked flask, stir, heat up to 65°C, and react After 3 hours, the reaction was completed, lowered to room temperature, and then slowly added dropwise a solution of 6.64g (207.5mmol) sulfur and 19.92g (83.0mmol) sodium sulfide nonahydrate in water (100mL) that had been prepared in advance. After the dropwise addition was completed, the temperature was raised React at 50°C for 6 hours. After the reaction is complete, cool down to room temperature. Adjust the pH of the system to 1-2 with 10% dilute phosphoric acid prepared in advance. A large amount of off-white solid precipitates. Keep stirring and crystallizing for 0.5 hours and then filter to collect the crude solid. , and then the solid crude product was placed in a 250 mL three-neck flask, recrystallized with an appropriate amount of ethanol and water, and finally 9.08 g of the target impurit...
Embodiment 2
[0026] Put 10.00g (41.5mmol) ethyl 6,8-dichlorooctanoate, (100mL) water, and 1.66g (41.5mmol) sodium hydroxide into a 500mL three-necked flask, stir, heat up to 65°C, and react After 3 hours, the reaction was completed, lowered to room temperature, and then slowly added dropwise a solution of 6.64g (207.5mmol) sulfur and 19.92g (83.0mmol) sodium sulfide nonahydrate in water (100mL) that had been prepared in advance. After the dropwise addition was completed, the temperature was raised React at 50°C for 6 hours. After the reaction is complete, cool down to room temperature. Adjust the pH of the system to 1-2 with 10% dilute phosphoric acid prepared in advance. A large amount of off-white solid precipitates. Keep stirring and crystallizing for 0.5 hours and then filter to collect the crude solid. , and then the solid crude product was placed in a 250 mL three-neck flask, recrystallized with an appropriate amount of ethanol and water, and finally 8.95 g of the target impurity was ...
Embodiment 3
[0028] Put 10.00g (41.5mmol) of ethyl 6,8-dichlorooctanoate, (100mL) water, and 1.74g (43.6mmol) of sodium hydroxide into a 500mL three-necked flask, stir, and raise the temperature to 65°C to react After 3 hours, the reaction was completed, lowered to room temperature, and then slowly added dropwise a solution of 6.64g (207.5mmol) sulfur and 14.95g (62.3mmol) sodium sulfide nonahydrate in water (100mL) that had been prepared in advance. After the dropwise addition was completed, the temperature was raised React at 50°C for 6 hours. After the reaction is complete, cool down to room temperature. Adjust the pH of the system to 1-2 with 10% dilute phosphoric acid prepared in advance. A large amount of off-white solid precipitates. Keep stirring and crystallizing for 0.5 hours and then filter to collect the crude solid. , and then the solid crude product was placed in a 250 mL three-neck flask, recrystallized with an appropriate amount of ethanol and water, and finally 9.26 g of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com