Carboxylic acid group-containing polymer, preparation method and application thereof
A technology of carboxylic acid groups and polymers, which is applied in the field of polymers containing carboxylic acid groups and their preparation and application, which can solve the problem of excellent, outstanding anti-redeposition ability, no report of inorganic stains and organic stain detergency, etc. problems, achieve good compatibility, improve detergency and anti-redeposition performance, and improve anti-redeposition performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
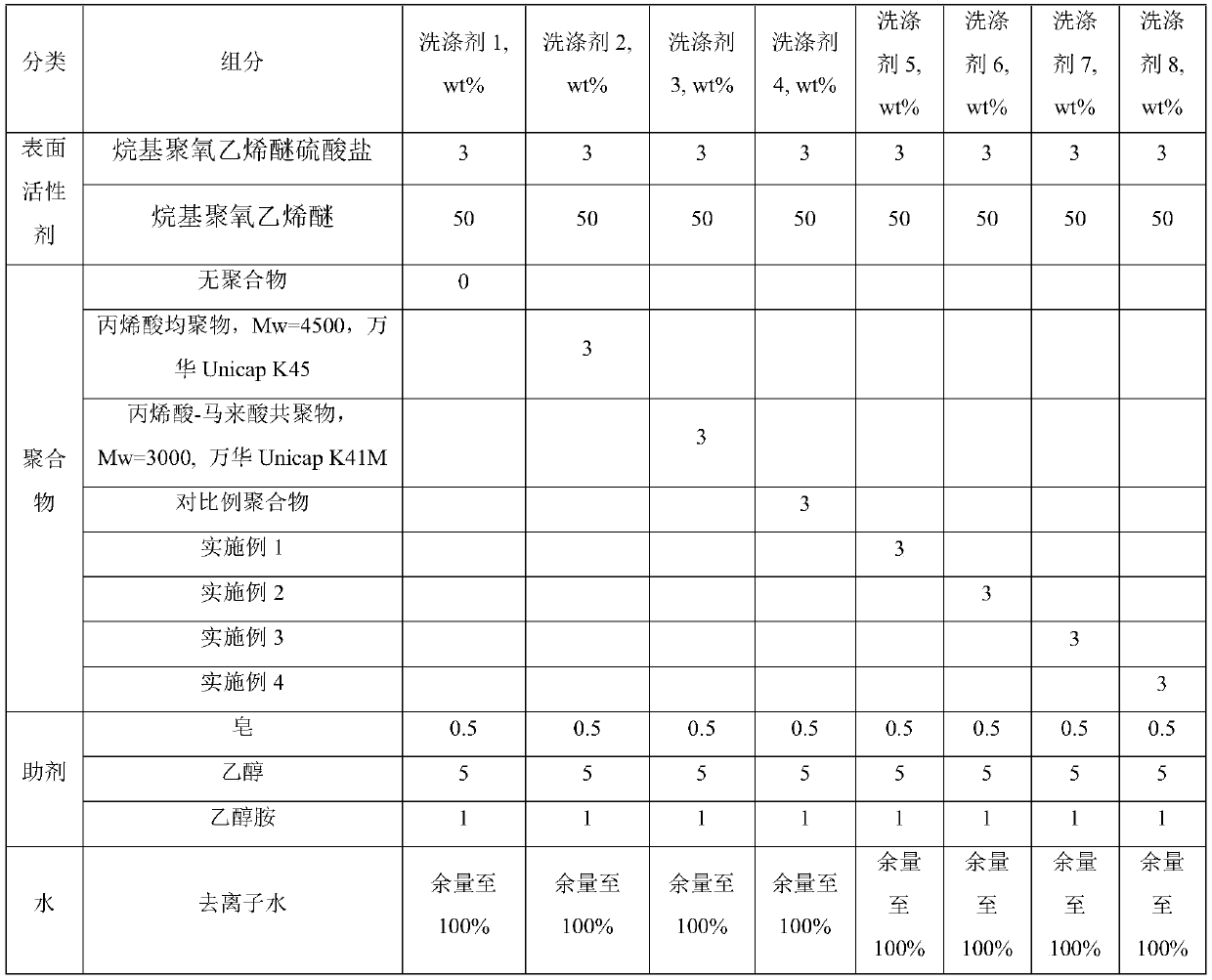
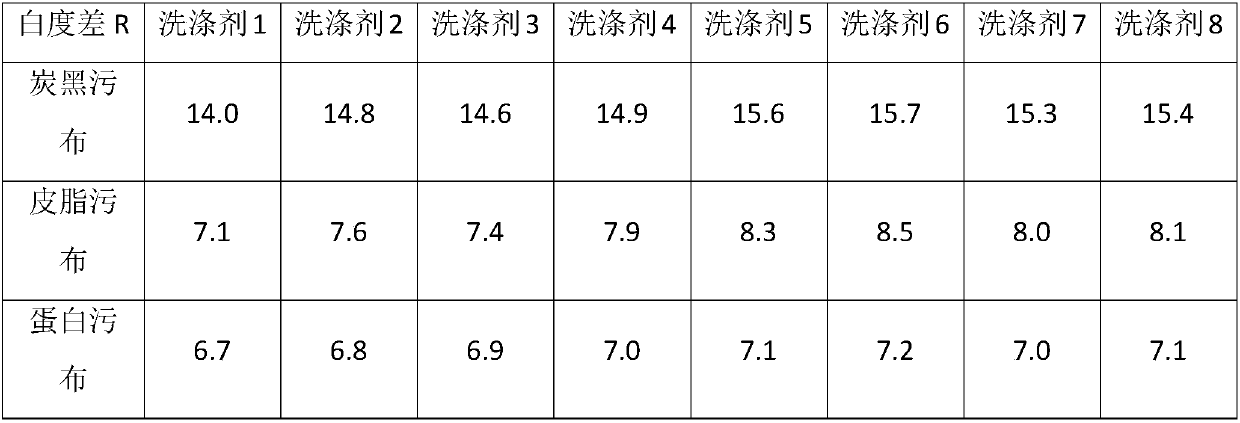
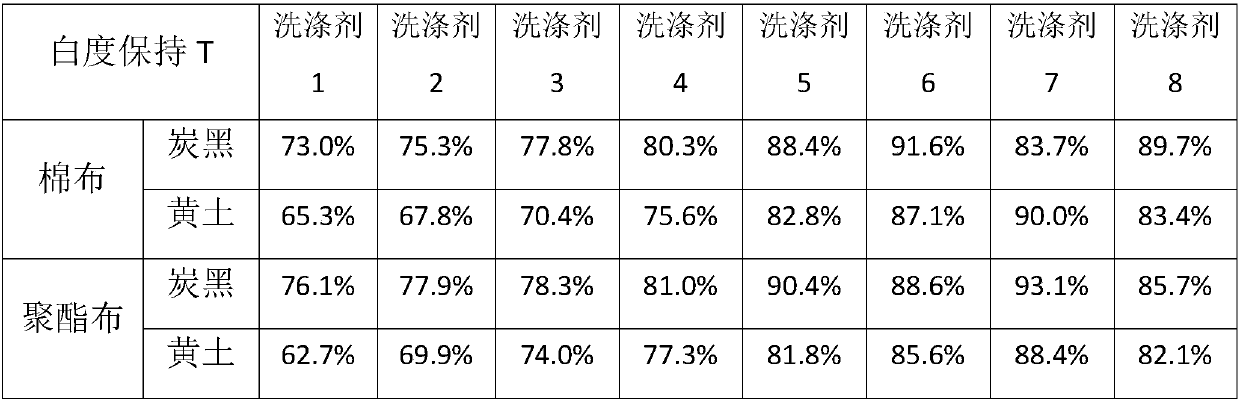
[0025] Below in conjunction with specific embodiment the present invention is further described, by embodiment, polycarboxylate polymer of the present invention can be prepared according to following embodiment 1-4:
Embodiment 1
[0027] In a 1L round-bottom four-neck flask equipped with a stirrer, a condenser and a feeding port, add 100 g of water, 150 g of isopropanol and 1.12 g of sodium persulfate, stir to dissolve and heat to 80°C. Prepare 58g of acrylic acid, 75g of lauryl acrylate and 24g of allyl alcohol polyoxyethylene polyoxypropylene ether (methyl-terminated, EO:PO=3:1, weight average molecular weight about 650) monomer mixture, prepare 4.48g of sodium persulfate And the initiator mixture of 15g water, prepare the mixture of 44g sodium hydroxide and 120g water. The first two mixtures were simultaneously added dropwise to the reactor at 80° C. for 4 hours, and kept warm for 1 hour after the dropwise addition. Then, the aqueous sodium hydroxide solution was added to the reactor by dropwise addition for 0.5 h. After the dropwise addition, isopropanol was removed by vacuum distillation, and deionized water was added to adjust the polymer aqueous solution to 40% solid content. Using GPC to test ...
Embodiment 2
[0029] In a 1L round-bottomed four-neck flask equipped with a stirrer, a condenser and a feeding port, add 87.5g of water, 162.5g of isopropanol and 1.44g of sodium persulfate, stir to dissolve and heat to 80°C. Prepare 72g acrylic acid, 64g styrene and 16g allyl alcohol polyoxyethylene polyoxypropylene ether (methyl-terminated, EO:PO=4:1, weight average molecular weight about 1000) monomer mixture, prepare 5.76g sodium persulfate and For an initiator mixture of 15g of water, prepare a mixture of 40g of sodium hydroxide and 120g of water. The first two mixtures were simultaneously added dropwise to the reactor at 80° C. for 4 hours, and kept warm for 1 hour after the dropwise addition. Then, the aqueous sodium hydroxide solution was added to the reactor by dropwise addition for 0.5 h. After the dropwise addition, isopropanol was removed by vacuum distillation, and deionized water was added to adjust the polymer aqueous solution to 40% solid content. Using GPC to test final p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com