Application of diacerein in preparation of antiviral drugs and treatment of virus infection
A virus infection and drug technology, applied in the direction of antiviral agents, resistance to vector-borne diseases, pharmaceutical formulations, etc., can solve problems such as diacerein antiviral reports that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
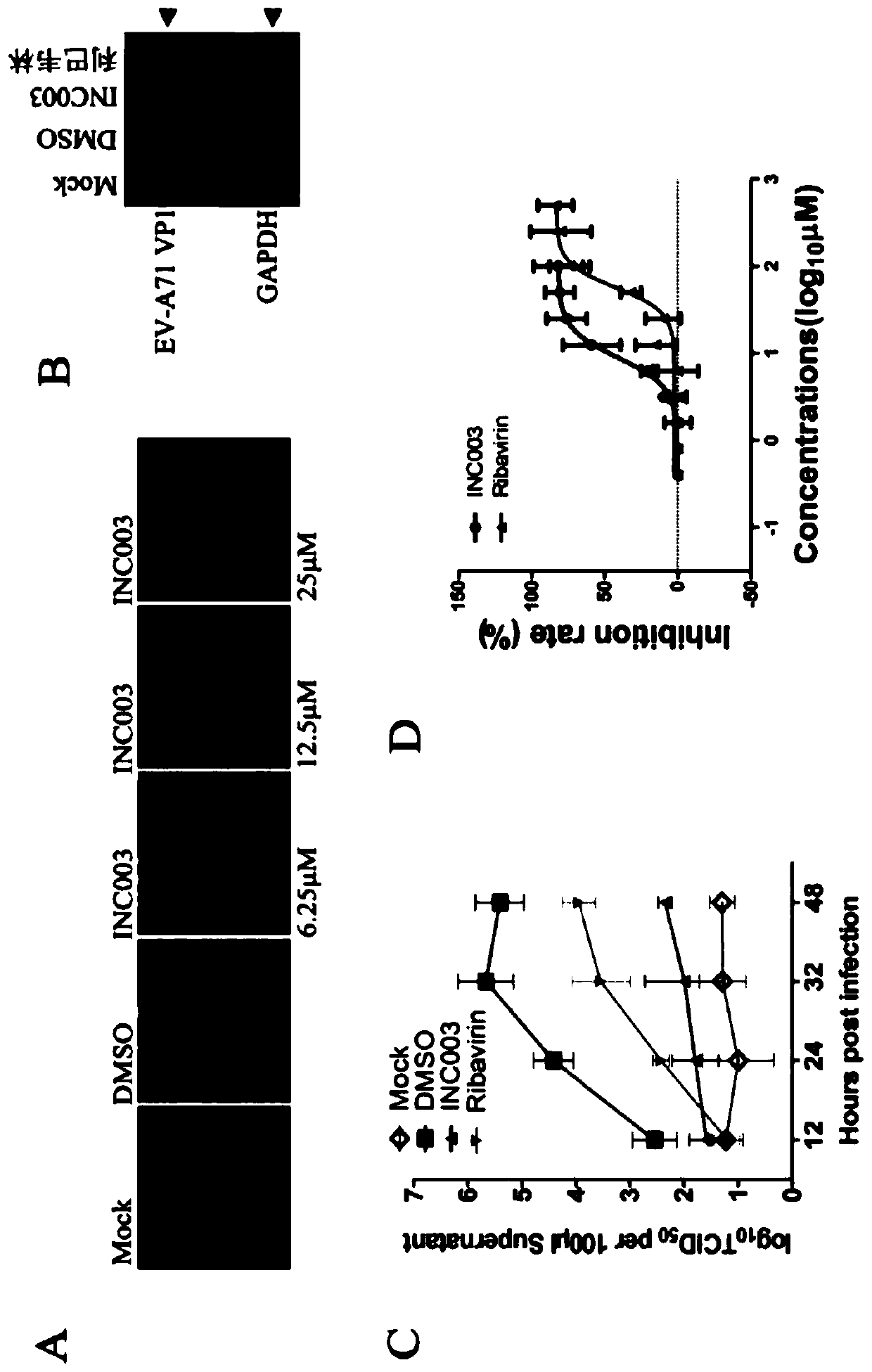
[0103] Exemplary Experimental Example 1. Cellular Pharmacodynamic Detection of the Inhibitory Effect of Diacerein on Human Enteroviruses
[0104]
[0105] 1. Cell culture, amplification and purification of EV-A71 virus
[0106] RD cells were cultured in DMEM medium containing 10% FBS. When the number of cells is sufficient, divide them into 10cm culture dishes with a density of 70%.
[0107] Put it in a 37-degree carbon dioxide incubator for cultivation. After 24 hours, dilute the virus with DMEM and discard the culture medium, add 4ml of virus diluent to each dish, and inoculate EV-A71 virus according to MOI=1. After adsorption at 37°C for 1 hour, the supernatant was discarded, and 10 ml of 5% FBS medium was added. Incubate at 37 degrees for 48 hours, and observe whether the cells have reached 50% detachment. The supernatant was collected and centrifuged at 2000 rpm for 10 minutes. Take the supernatant.
[0108] Prepare 5×PEG8000 NaCl solution. Preparation Weigh 8.7...
experiment example 2
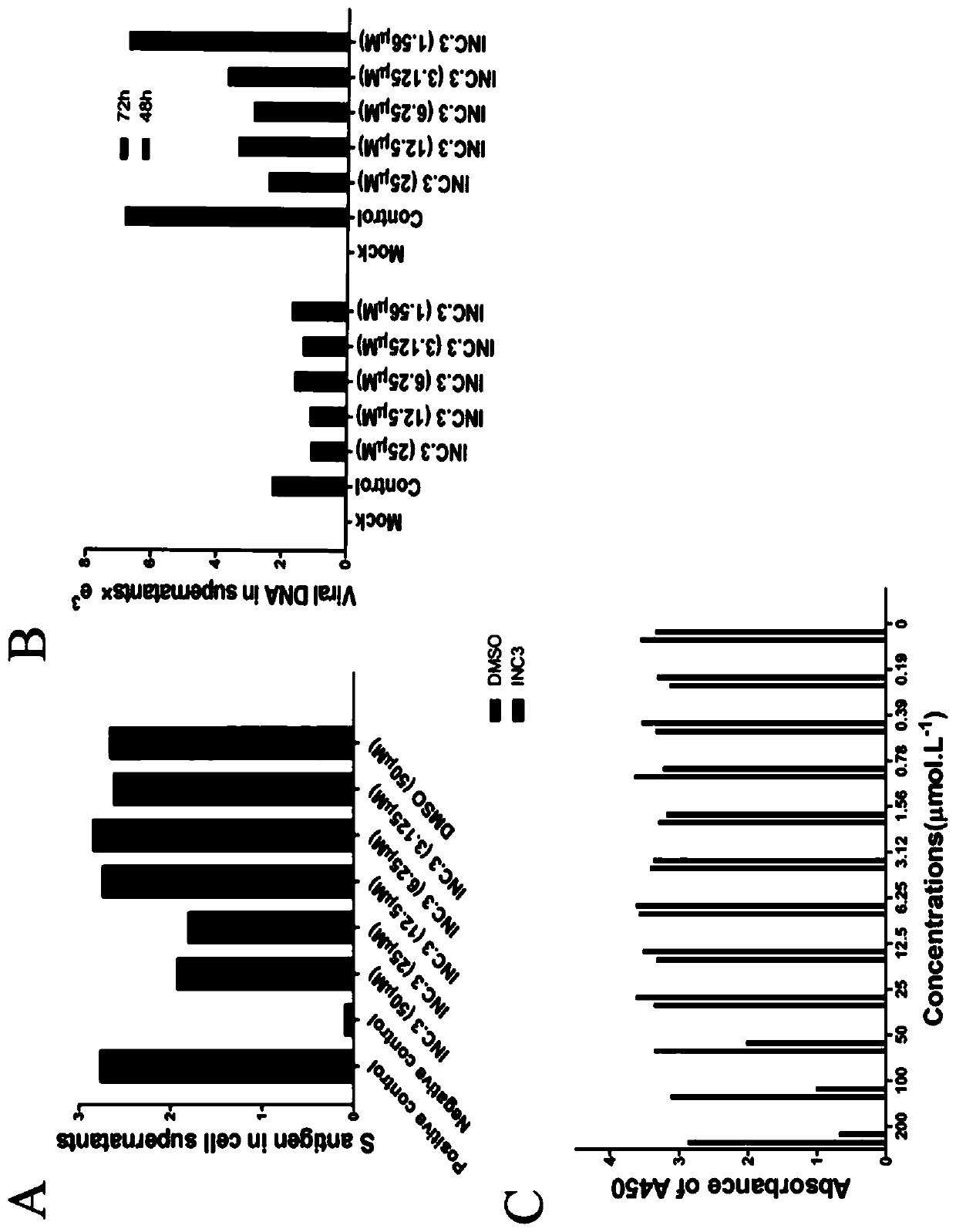
[0133] Exemplary Experiment 2. Cell-level Pharmacodynamic Detection of Diacerein's Inhibitory Effect on Hepatitis B Virus
[0134]
[0135] 1. Elisa assay was used to detect hepatitis B virus S antigen in the supernatant of the hepatitis B HepG2-2215 cell model.
[0136] The supernatant of HepG2-2215 cells treated with diacerein was aspirated, and the DMSO group was used as the control. According to the instructions of the detection kit, the pre-experiment tests the different dilution ratios of the cell supernatant, in order to meet the different dilution ratios within the range of the instrument.
[0137] The specific detection method is as follows:
[0138] Add the diluted cell supernatant to the enzyme-labeled wells, 100 microliters per well.
[0139] Incubate at 37 degrees for 1 hour to allow the S antigen to bind to the antibody (the antibody on the microplate in the kit).
[0140] Wash three times with wash solution, pat dry each time. Add 50 microliters of chromo...
experiment example 3
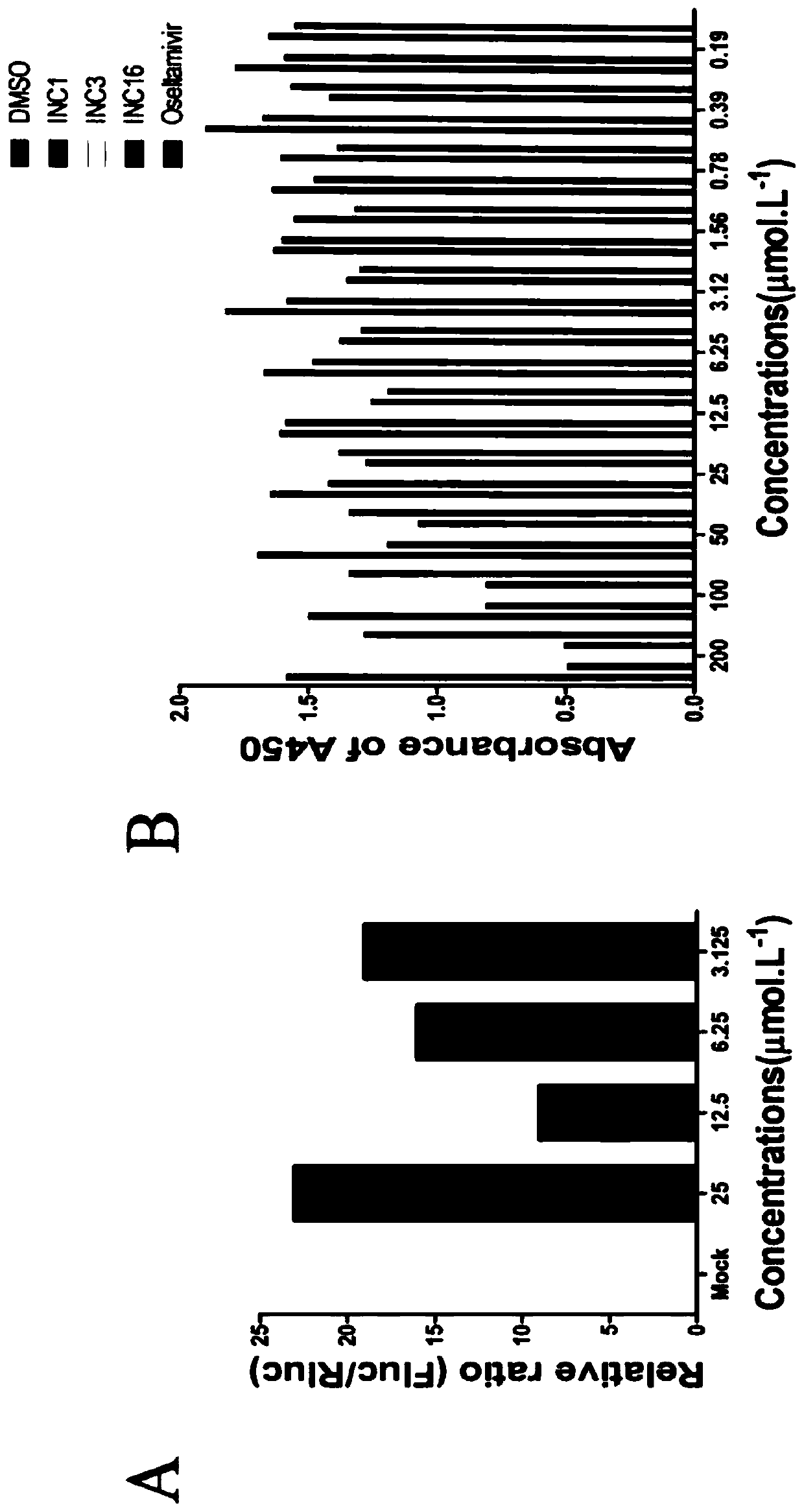
[0157] The result is as figure 2 As shown in C, diacerein does not cause significant cytotoxicity at concentrations below 25 μM. exemplary experiment Example 3. Cell-level pharmacodynamic detection of the inhibitory effect of diacerein on human immunodeficiency virus (HIV-1)
[0158]
[0159] 1. Packaging of HIV-1 pseudovirus
[0160] 293T cells were cultured in 10% FBS DMEM medium. The plasmids of pNL4-3 Luc-R-E and VSVG were extracted in large quantities, and the concentration and purity of the plasmids were measured by Nanodrop. The cells in better state were digested and divided into 10cm culture dishes. After culturing for 24 hours, the medium was discarded, rinsed twice with DMEM, and 5 ml of DMEM was added. Mix pNL4-3 Luc-R-E and VSVG medium 1:1 by mole ratio (the total mass is 6 micrograms), and add 60 microliters of PEI transfection reagent. Transfect 293T cells. After incubating at 37 degrees for 2 hours, add 4 ml of DMEM. The incubation was continued f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com