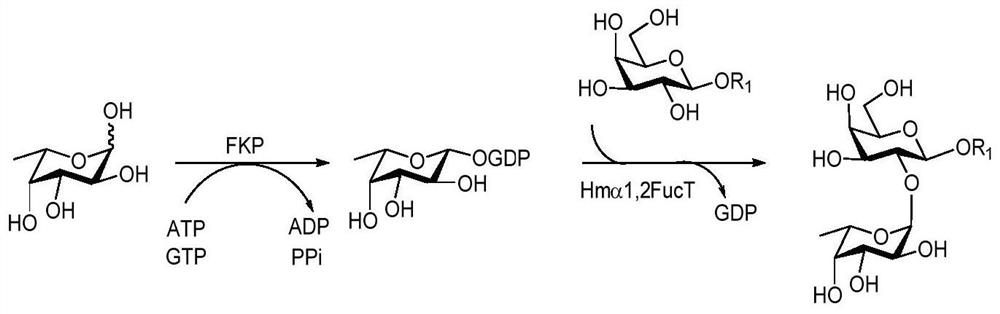
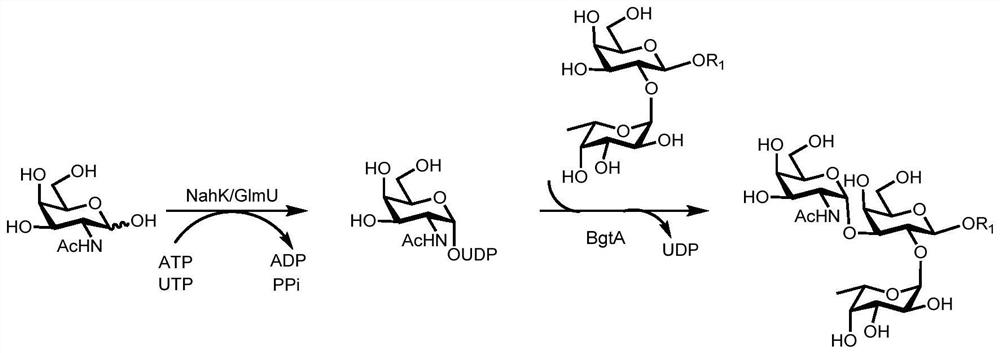
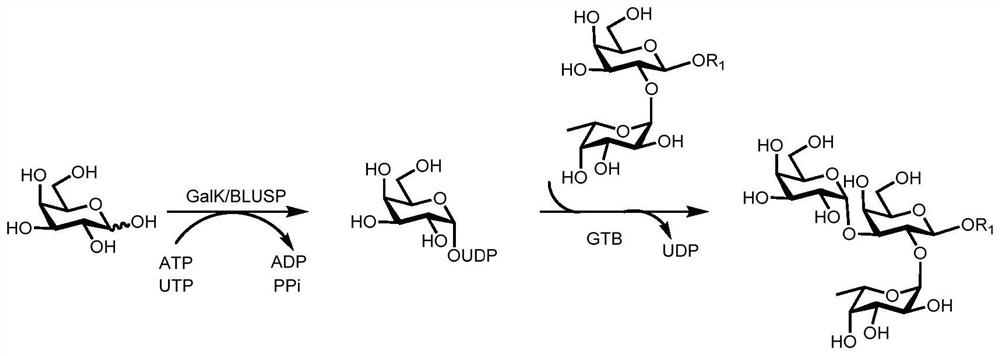
A kind of synthesis method of galnacα1,3gal or galα1,3gal glycosidic bond oligosaccharide
A synthetic method and glycosidic bond technology, applied in biochemical equipment and methods, glycosyltransferase, transferase, etc., can solve problems such as poor substrate tolerance, limited substrate adaptability, and difficulty in separation and purification, and achieve High assembly efficiency, wide substrate adaptability, and improved overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Synthesis of oligosaccharides containing GalNAcα1,3Gal or Galα1,3Gal glycosidic bonds
[0068] Proceed as follows:
[0069] (1) Enzymatic synthesis of disaccharide compound 1[Galβl,3GlcNAcβProN 3 ]
[0070] Compound 1 of the present invention [Galβl, 3GlcNAcβProN 3 ] The synthetic method is as follows:
[0071] Add receptor GlcNAcβProN to 50mL centrifuge tube 3 (0.10g, 0.33mmol), galactose (0.09g, 0.50mmol), adenosine triphosphate (ATP, 0.27g, 0.50mmol), Tris-HCl (100mmol, pH 7.5) and magnesium chloride (20mmol), dissolved In a little three-distilled water, shake until completely dissolved, adjust the pH to about 7.0 with 1mol / L HCl or 1mol / L NaOH, then add the enzyme GalK (2.00mg), BiGalHexNAcP (1.50mg), and finally decompose the reaction with three-distilled water The total volume of the liquid was added to 10mL, and reacted for 11h at 37°C under the condition of 100r / min, and the reaction progress was determined by thin-layer chromatography (EtOAc:MeO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com