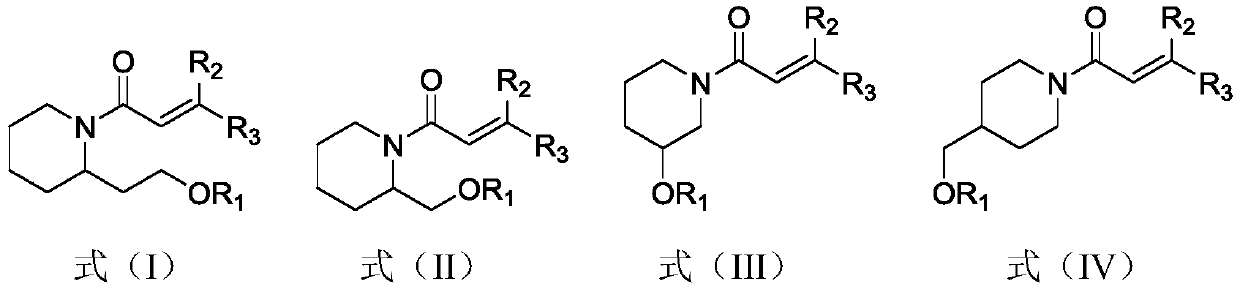
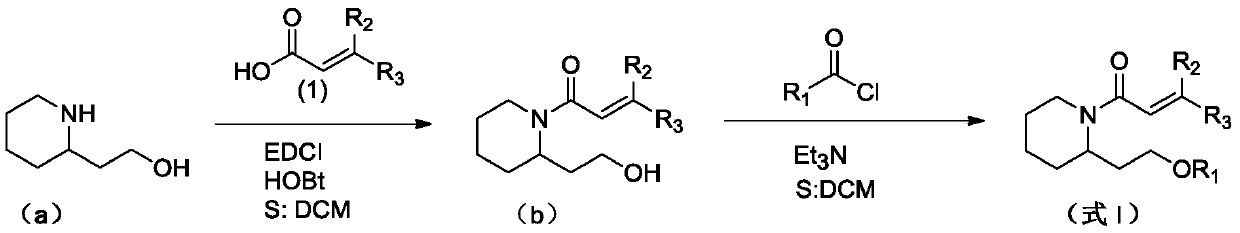
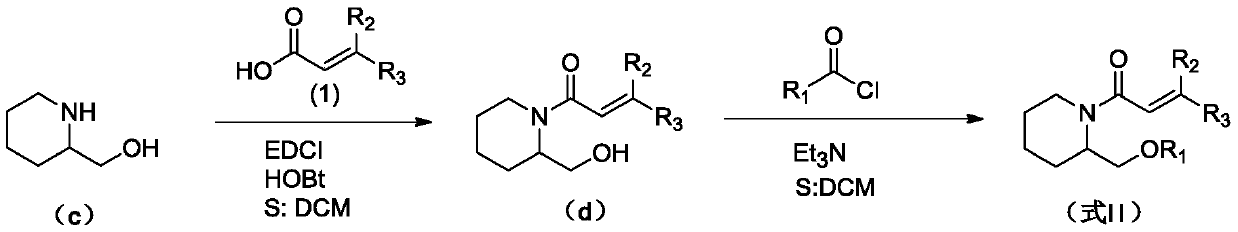
(Trans)-beta-farnesene analogue containing hydroxypiperidine as well as preparation and application thereof
A technology of hydroxypiperidine and farnesene, which is applied in the fields of application, pest control, and pest repellent, and can solve problems such as drug resistance and ecological environment pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of (trans)-β-farnesene analogs containing hydroxypiperidine
[0029] Add 0.57g (2.97mmol) of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, 0.40g (2.97mmol) 1-hydroxybenzotriazepam in a 50mL three-necked flask azole and 0.5g (2.97mmol) acrylic acid or geranic acid. Add 15 mL of dichloromethane solution to dissolve, and stir at room temperature for 1 hour. The above solution was added dropwise into a 100 mL three-neck flask containing 0.38 g of 2-hydroxyethylpiperidine (compound represented by formula a) and 20 mL of dichloromethane solution under ice cooling. After the dropwise addition, the reaction was carried out at room temperature for 2 hours. Wash the reaction solution with water, extract with dichloromethane, combine the organic phases, and wash with MgSO 4 dry. After concentration, column chromatography (petroleum ether: ethyl acetate (volume ratio) = 2:1) gave the target compound (compound represented by formula b) as a l...
Embodiment 2
[0054] Example 2: Repellent activity of hydroxypiperidine-containing (trans)-β-farnesene analogues against aphids
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com