Fully enclosed cell culture method
一种细胞培养、全封闭的技术,应用在细胞培养领域,能够解决失误、大人力成本及时间成本、不利细胞生长等问题,达到降低成本、减少人工操作、高细胞培养密度的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
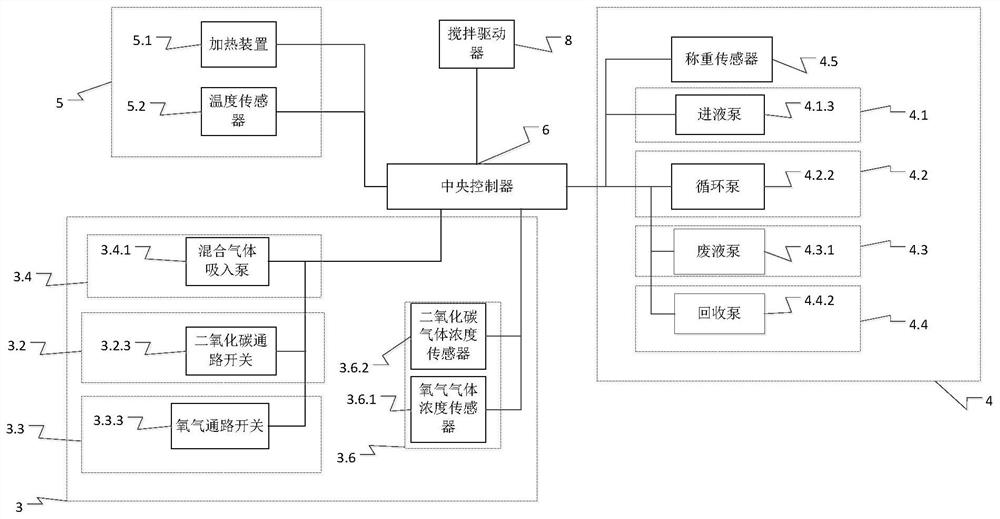
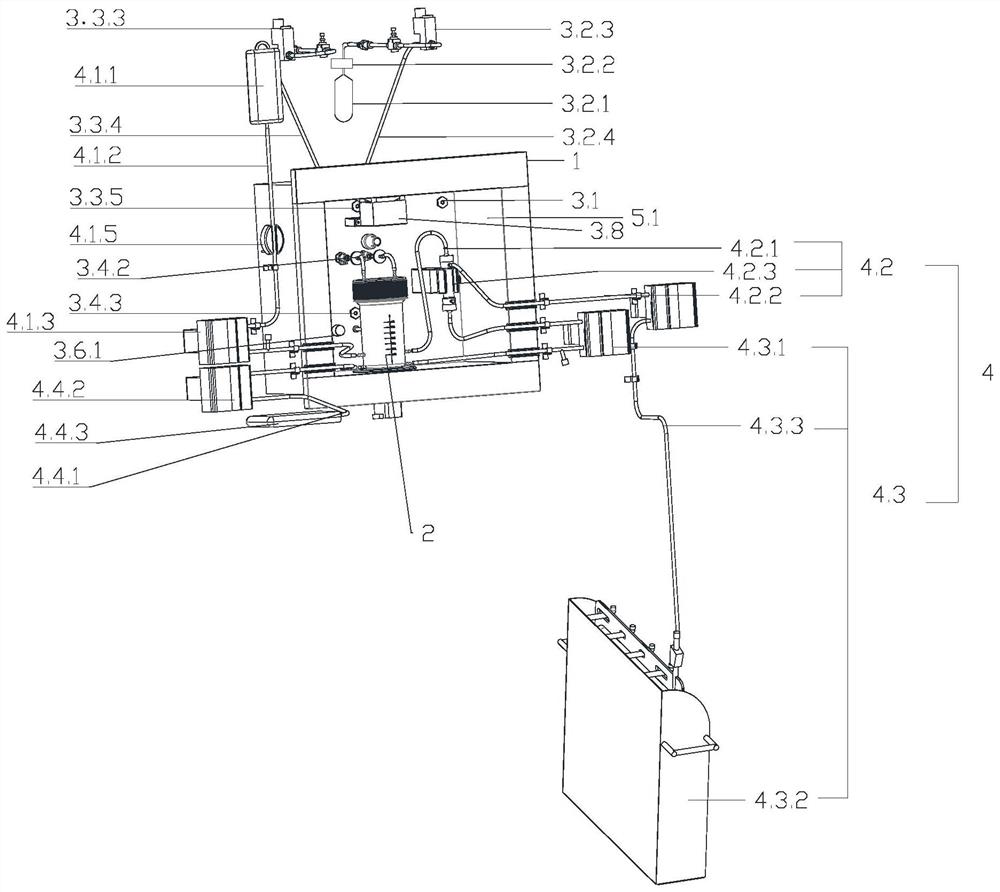
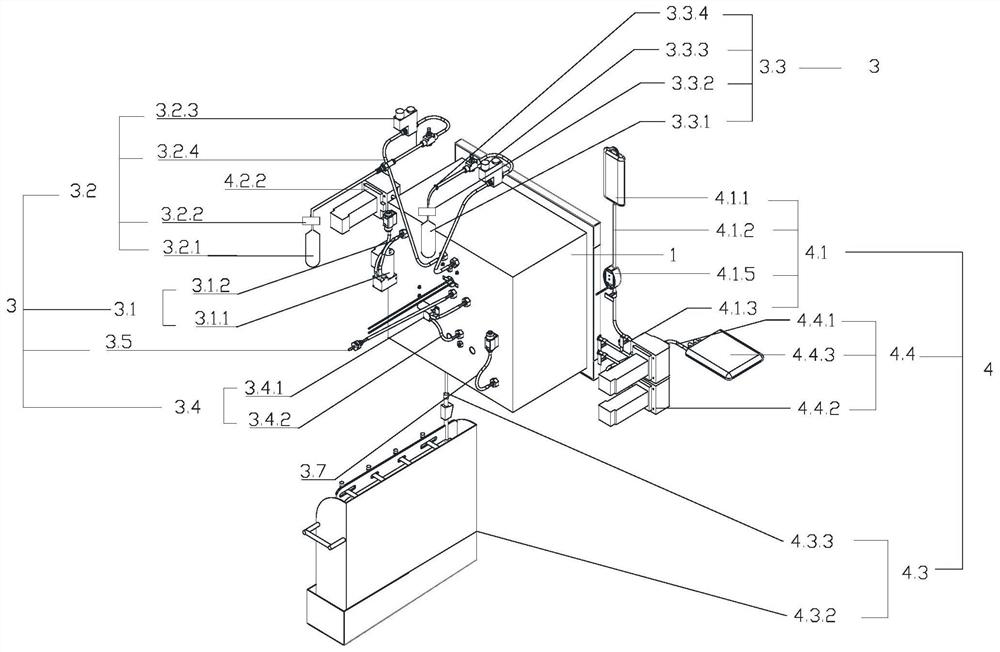
Image
Examples
Embodiment 1
[0246] According to the technological process and parameters in Table 1, an embodiment of the present invention adopts the fully enclosed cell culture method of the present invention to cultivate CAR-T cells (hereinafter referred to as CCS culture), and compares with the traditional manual CAR-T cell culture method (compared to group) for comparison. specific:
[0247] 1.1 Culture method and results:
[0248] CCS culture process of CAR-T cells: On the first day, 1E+09 PBMC cells were separated and sorted, and CD3 / CD28 was added for activation; on the second day, when the total number of cells was not less than 1E7, the infection was carried out. The infection protocol: Infection density 2E5-1E6 / ml, MOI 1-10; cell expansion from the third day to the tenth day, stirring speed 20-100r / min, rehydration by perfusion: circulation pump speed 20-150r / min, perfusion time 40-60min; Harvest the cells on the eleventh day: Concentrate the cell suspension to 100-200ml, perform perfusion r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com