Pyridine-bridged double-ruthenium acetylene terminal group compound and preparation method and application thereof
A pyridine bridged and compound technology is applied in the field of pyridine bridged bisruthenium acetylene end-group compounds and the preparation thereof, can solve problems such as the synthesis method of bisruthenium acetylene end-group compounds that have not been seen, and achieves good electronic interaction, good The effect of charge transport properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
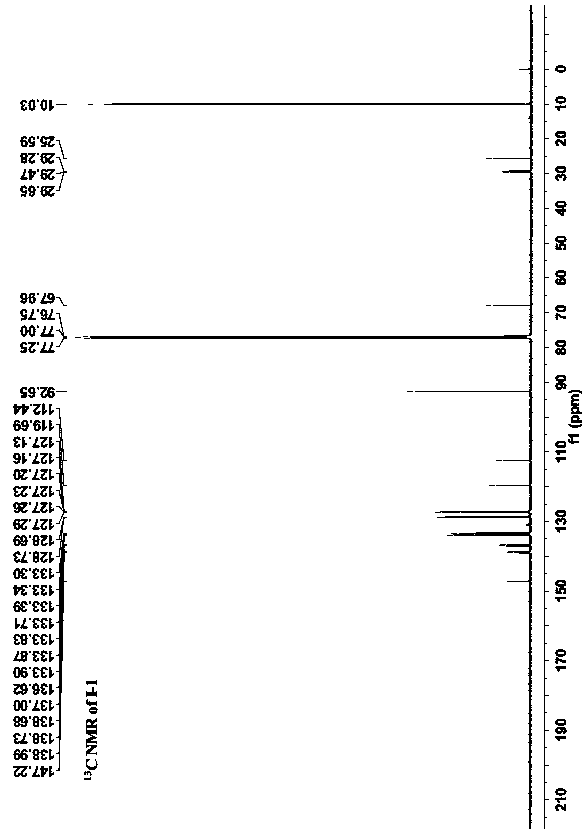
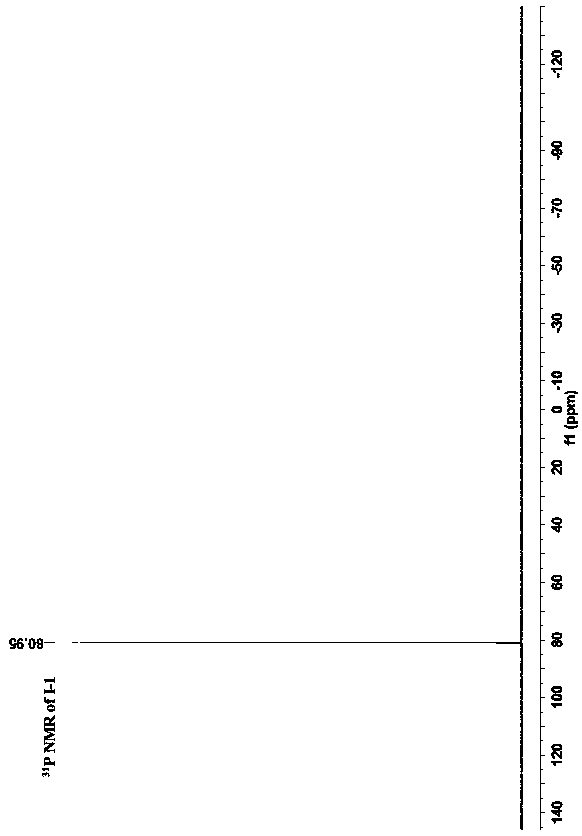
[0039] Preparation of pyridine-bridged double ruthenium acetylene terminal compounds: under nitrogen protection, an appropriate amount of pentamethylcyclopentadienyl (1,2-bisdiphenylphosphineethane) ruthenium chloride Cp * Ru(dppe)Cl (preferably 500mg, 0.74mmol), 2,6-bis(trimethylsilylethynyl)pyridine (preferably 80mg, 0.30mmol) and potassium fluoride (preferably 205mg, 3.54mmol) were dissolved in an appropriate amount of methanol ( preferably 20ml) and tetrahydrofuran (preferably 3-4ml), the system is heated to reflux until the reaction is complete (about 24 hours), cooled to room temperature, suction filtered, and the solids are washed with 5-10ml methanol and 5-10ml n-hexane respectively , and then recrystallized with dichloromethane and n-hexane to obtain 250 mg of a brown solid with a yield of 57%. In the obtained pyridine-bridged double ruthenium acetylene terminal compound, R' is H, and the structural formula is: Its NMR spectrum is shown in Figure 1-3 shown.
[004...
Embodiment 2
[0046] Preparation of pyridine-bridged double ruthenium acetylene terminal compounds: under nitrogen protection, an appropriate amount of pentamethylcyclopentadienyl (1,2-bisdiphenylphosphineethane) ruthenium chloride Cp * Ru(dppe)Cl (preferably 500mg, 0.74mmol), 3,5-bis(trimethylsilylethynyl)pyridine (preferably 80mg, 0.30mmol) and potassium fluoride (preferably 205mg, 3.54mmol) were dissolved in an appropriate amount of methanol ( preferably 20ml) and tetrahydrofuran (preferably 3-4ml), the system is heated to reflux until the reaction is complete (about 24 hours), cooled to room temperature, suction filtered, and the solids are washed with 5-10ml methanol and 5-10ml n-hexane respectively , and then recrystallized with dichloromethane and n-hexane to obtain 267 mg of brown solid with a yield of 61%, and the structural formula of the double ruthenium acetylene terminal compound bridged by the gained pyridine is: Its NMR spectrum is shown in Figure 4-6 shown.
[0047] Elem...
Embodiment 3
[0052] Preparation of pyridine-bridged double ruthenium acetylene terminal compounds: under nitrogen protection, an appropriate amount of pentamethylcyclopentadienyl (1,2-bisdiphenylphosphineethane) ruthenium chloride Cp * Ru(dppe)Cl (preferably 500mg, 0.74mmol), 2,5-bis(trimethylsilylethynyl)pyridine (preferably 80mg, 0.30mmol) and potassium fluoride (preferably 205mg, 3.54mmol) were dissolved in an appropriate amount of methanol ( preferably 20ml) and tetrahydrofuran (preferably 3-4ml), the system is heated to reflux until the reaction is complete (about 24 hours), cooled to room temperature, suction filtered, and the solids are washed with 5-10ml methanol and 5-10ml n-hexane respectively , and then recrystallized with dichloromethane and n-hexane to obtain 289 mg of brown solid with a yield of 61%, and the structural formula of the double ruthenium acetylene terminal compound bridged by the gained pyridine is: Its NMR spectrum is shown in Figure 7-9 shown.
[0053] Elem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com