Hole transport material, and preparation method and application thereof
A technology of hole transport material and hole transport layer, applied in the field of solar cells, can solve the problems of serious environmental hazards, reduced battery stability, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
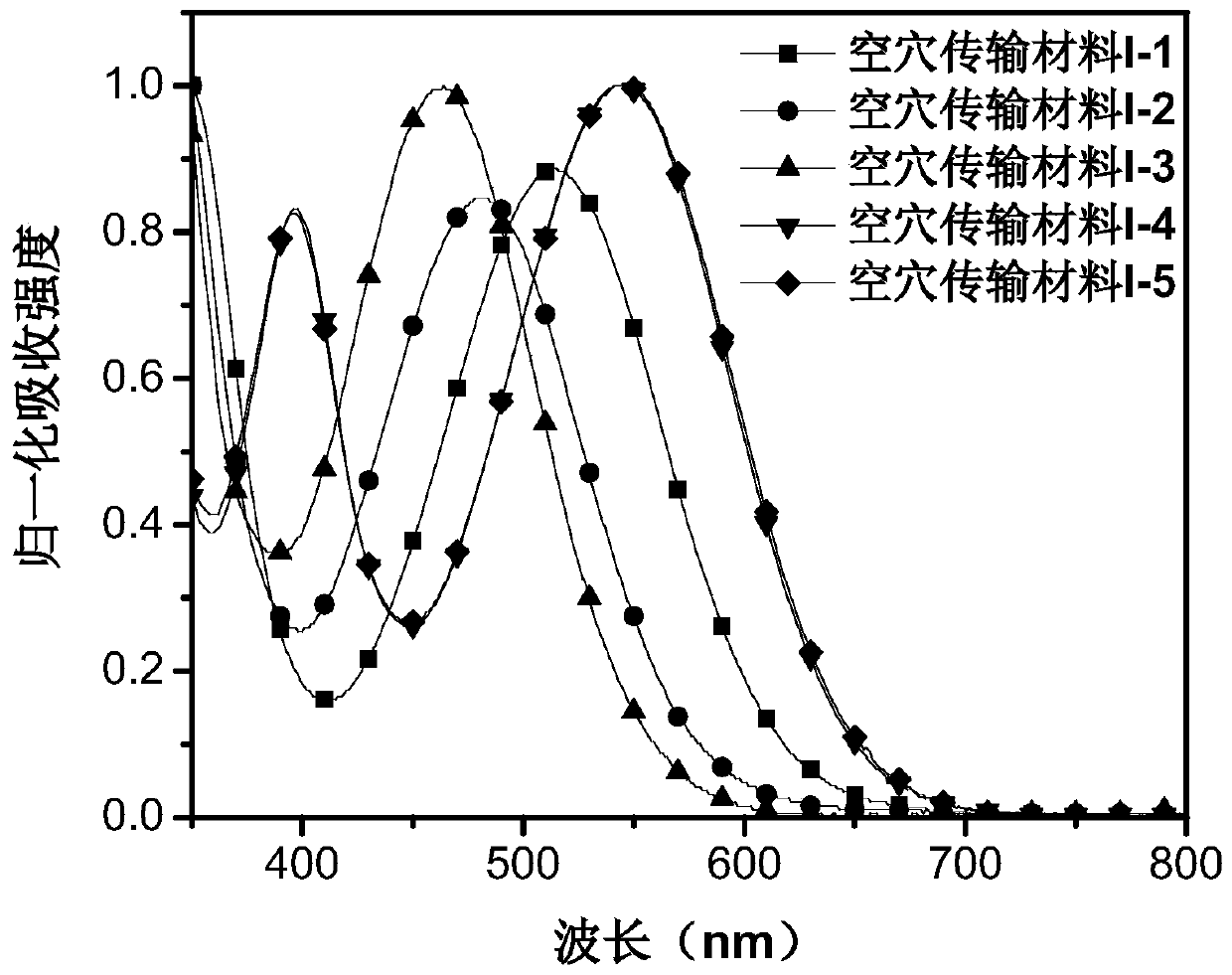
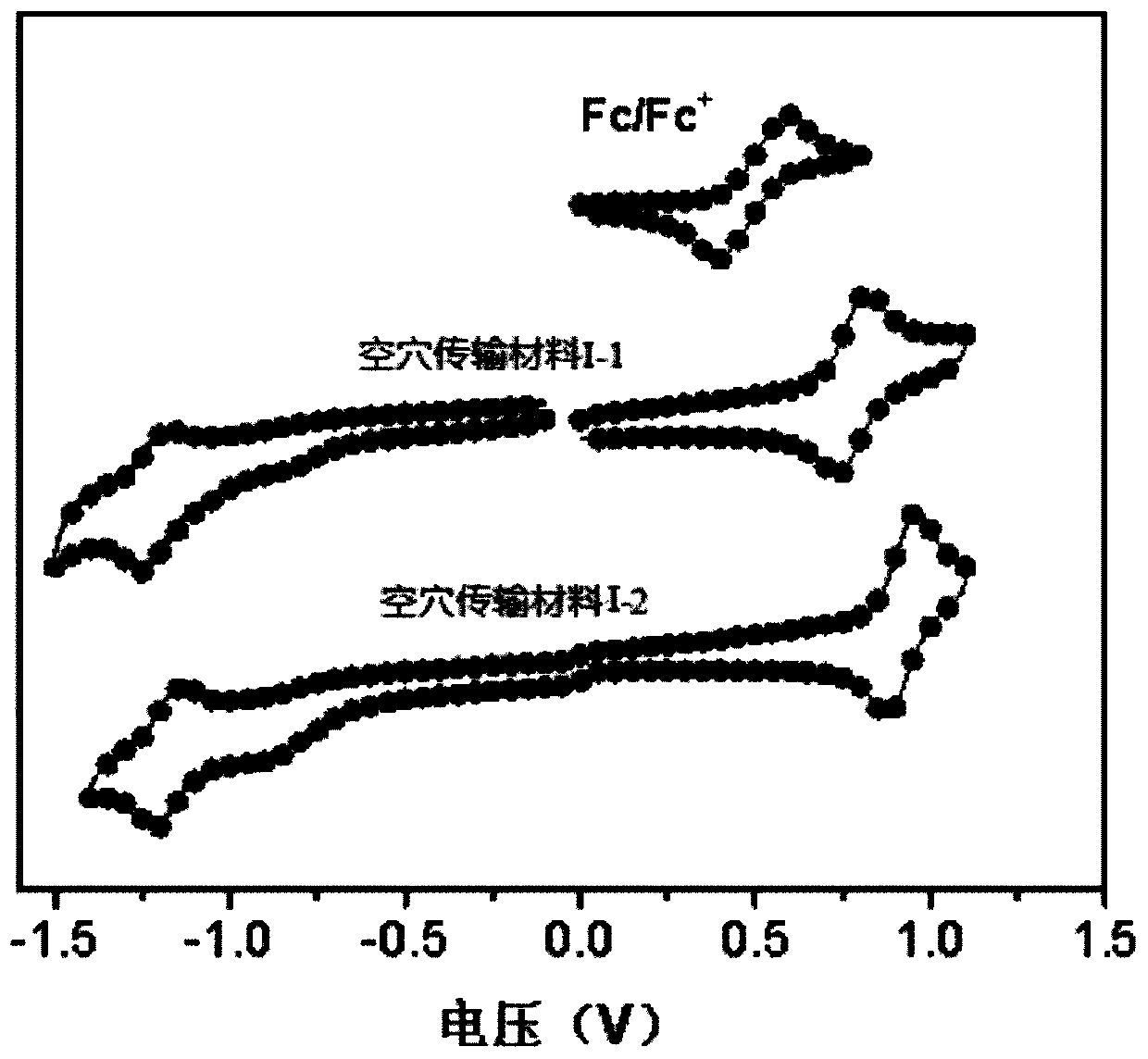
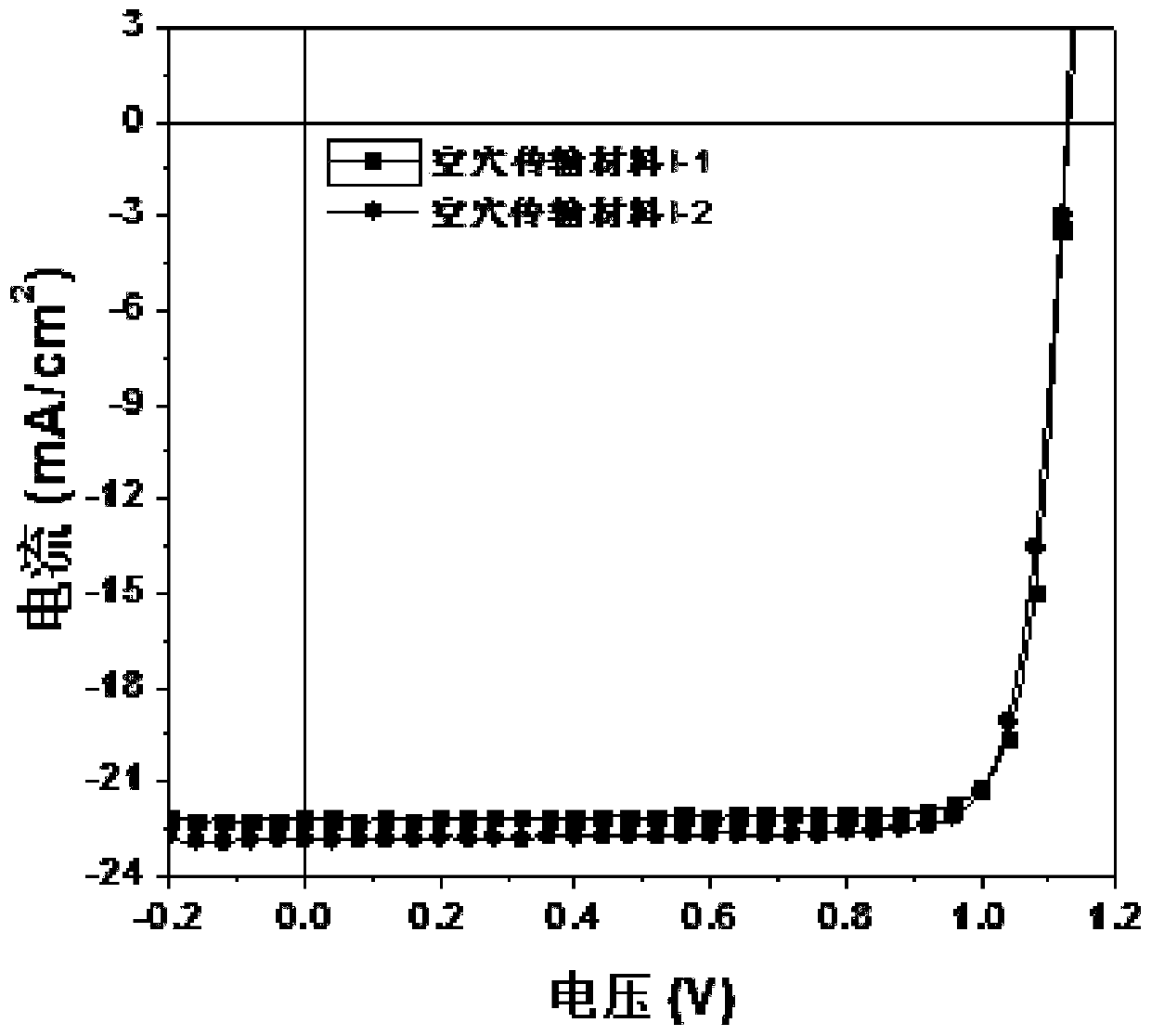
[0081] This embodiment provides a hole transport material, which has the structure shown in Formula I-1:
[0082]
[0083] Synthetic route of hole transport material Ⅰ-1:
[0084]
[0085] (1) Synthesis of intermediate compound 1
[0086] 4-(4-Methoxyphenylamino)phenylboronic acid pinacol ester (226mg, 0.52mmol), 7-bromo-4-formylbenzo[C][1,2,5]thiadiazole ( 100mg, 0.4mmol), Pd (PPh 3 ) 4 (25mg, 0.04mmol), potassium carbonate (90mg, 0.6mmol), placed in a two-necked bottle; after pumping argon three times, add 7mL of tetrahydrofuran and 1mL of water; the reaction was carried out under the protection of argon; reacted at 100°C for 24h Finally, after the reaction was completed, the reaction system was cooled to room temperature, the solvent was spun out, and the initial product was purified by column to obtain intermediate compound 1 with a mass of 170 mg and a yield of 91%.
[0087] 1 H NMR (400MHz, CDCl3): δ10.75(s, 1H), 8.28(d, J=7.5Hz, 1H), 7.92(d, J=8.9Hz, 2H), 7.8...
Embodiment 2
[0096] This embodiment provides a hole transport material, which has the structure shown in Formula I-2:
[0097]
[0098] Synthetic route of hole transport material Ⅰ-2:
[0099]
[0100] (1) Synthesis of compound 2: 4,4'-dimethoxydiphenylamine (960mg, 4.2mmol), 2-bromo-5-iodofluorobenzene (1.56g, 5.25mmol), Pd 2 (dba) 3 (193mg, 0.21mmol), dppf (112mg, 0.18mmol), sodium tert-butoxide (2g, 21mmol), placed in a two-necked bottle; after pumping argon for three times, add anhydrous toluene 25mL; react under the protection of argon After reacting at 120° C. for 24 hours, the reaction system was cooled to room temperature, the solvent was spun out, and the initial product was further purified through a column to obtain compound 2 with a mass of 1.5 g and a yield of 89%.
[0101] 1 H NMR (400MHz, CDCl3): δ7.26-7.23 (m, 1H), 7.08 (d, J = 8.8Hz, 4H), 6.87 (d, J = 8.8Hz, 4H), 6.64 (dd, J = 11.4 ,2.5Hz,1H),6.56(dd,J=8.8,2.5Hz,1H),3.82(s,6H).
[0102] (2) Synthesis of Compound...
Embodiment 3
[0111] This embodiment provides a hole transport material, which has the structure shown in Formula I-3:
[0112]
[0113] The synthetic route of hole transport material Ⅰ-3:
[0114]
[0115] (1) Synthesis of Compound 5: Compound 5 was synthesized according to the method for compound 2 in Synthesis Example 2, with a yield of 72%.
[0116] 1 H NMR (400MHz, CDCl3): δ7.30-7.26 (m, 1H), 7.12 (d, J = 8.2Hz, 4H), 7.02 (d, J = 8.4Hz, 4H), 6.74 (dd, J = 11.2 ,2.6Hz,1H),6.66(dd,J=8.8,2.6Hz,1H),2.35(s,6H).
[0117] 19 F NMR (376MHz, CDCl3): δ-106.79.
[0118] (2) Synthesis of Compound 6: Compound 6 was synthesized according to the method for compound 3 in Synthesis Example 2, with a yield of 88%.
[0119] 1 H NMR (400MHz, CDCl3): δ7.54-7.50 (m, 1H), 7.12 (d, J = 8.1Hz, 4H), 7.04 (d, J = 8.3Hz, 4H), 6.71 (dd, J = 8.3 ,2.0Hz,1H),6.58(dd,J=12.2,1.9Hz,1H),2.35(s,6H),1.36(s,12H).
[0120] 19 F NMR (376MHz, CDCl3): δ-101.74.
[0121] (3) Synthesis of Compound 7: Compound 7 wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com