Salicylamide compound preparation method and application thereof
A salicylamide and compound technology, applied to a class of salicylamide compounds, their preparation and application fields, can solve the problems of single action target, many toxic and side effects, poor long-term efficacy in AD patients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
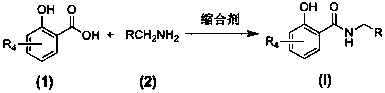
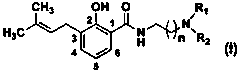
[0037] The preparation general method of embodiment 1 salicylamide compound (I)
[0038] Add 2.0 mmol of the corresponding salicylic acid compound (1), 3.0 mmol of the corresponding primary amine compound (2) and 15 ml of tetrahydrofuran in sequence in the reaction flask, stir well at room temperature and then add 1-ethyl-3-(3- Dimethylaminopropyl) carbodiimide hydrochloride 3.0 mmol and triethylamine 4.0 mmol, continue stirring at room temperature for 10 to 32 hours (reaction progress tracked by TLC). After the reaction was completed, the solvent was evaporated under reduced pressure, 30 mL of dichloromethane was added to the residue, followed by 20 mL of deionized water, 20 mL of saturated NaHCO 3 aqueous solution and 20 mL saturated NaCl aqueous solution, the organic layer was dried over anhydrous sodium sulfate and filtered, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography (eluent: petroleum ether: aceto...
Embodiment 2
[0087] Example 2 Salicylamide Compound (I) and Acid Salt Preparation General Method
[0088] Add 2.0 mmol of the salicylamide compound (I) obtained according to the above-mentioned Example 1 and 15 ml of acetone into the reaction flask, stir evenly, add 3.0 mmol of the corresponding acid, heat up and reflux and stir for 20 minutes, and cool to room temperature after the reaction is completed. The solvent was distilled off under reduced pressure, and the residue was recrystallized to obtain the salt of salicylamide compound (I) and acid. 1 Confirmed by HNMR and ESI-MS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com