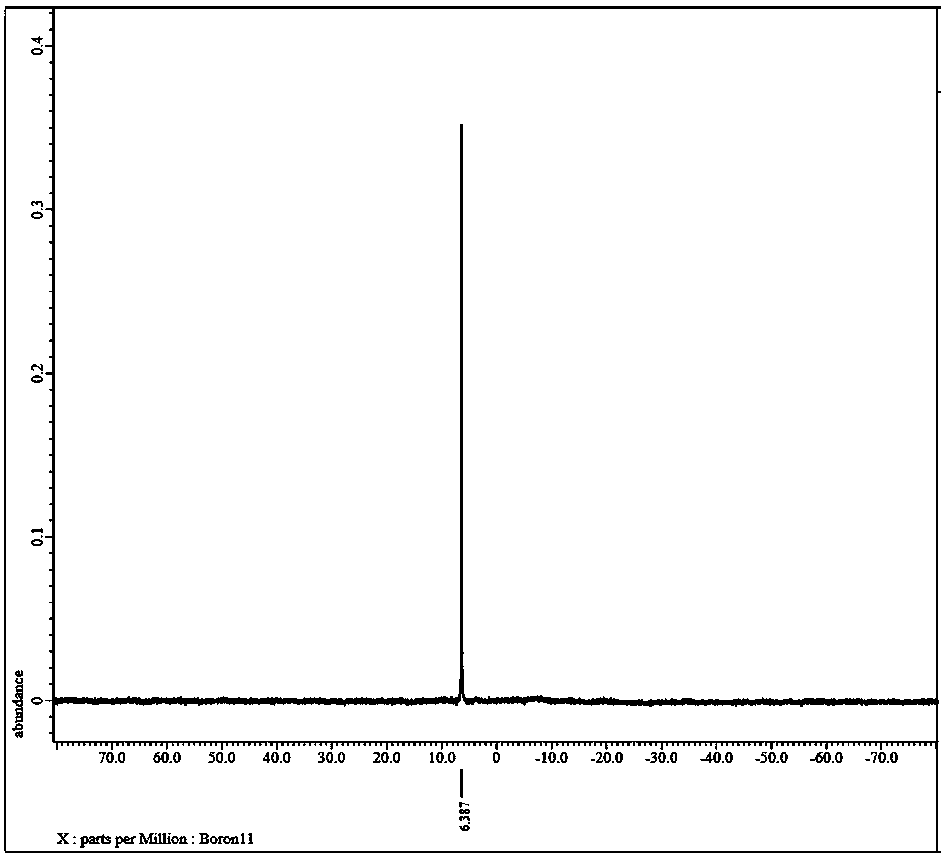
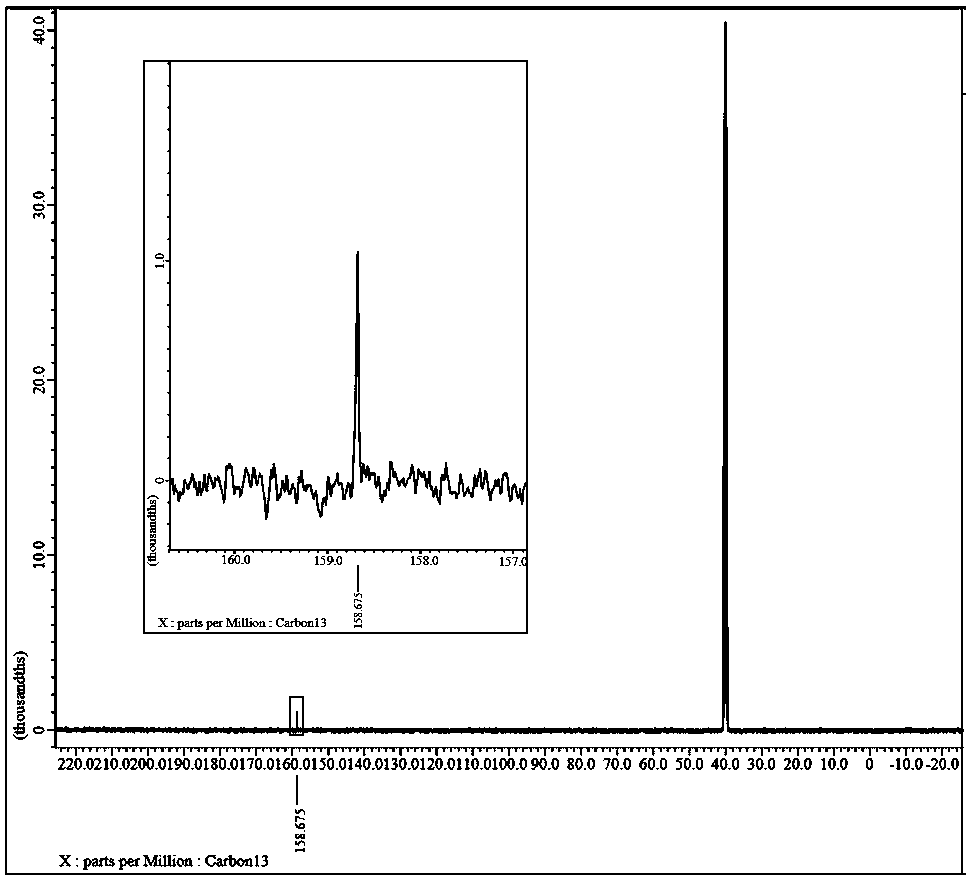
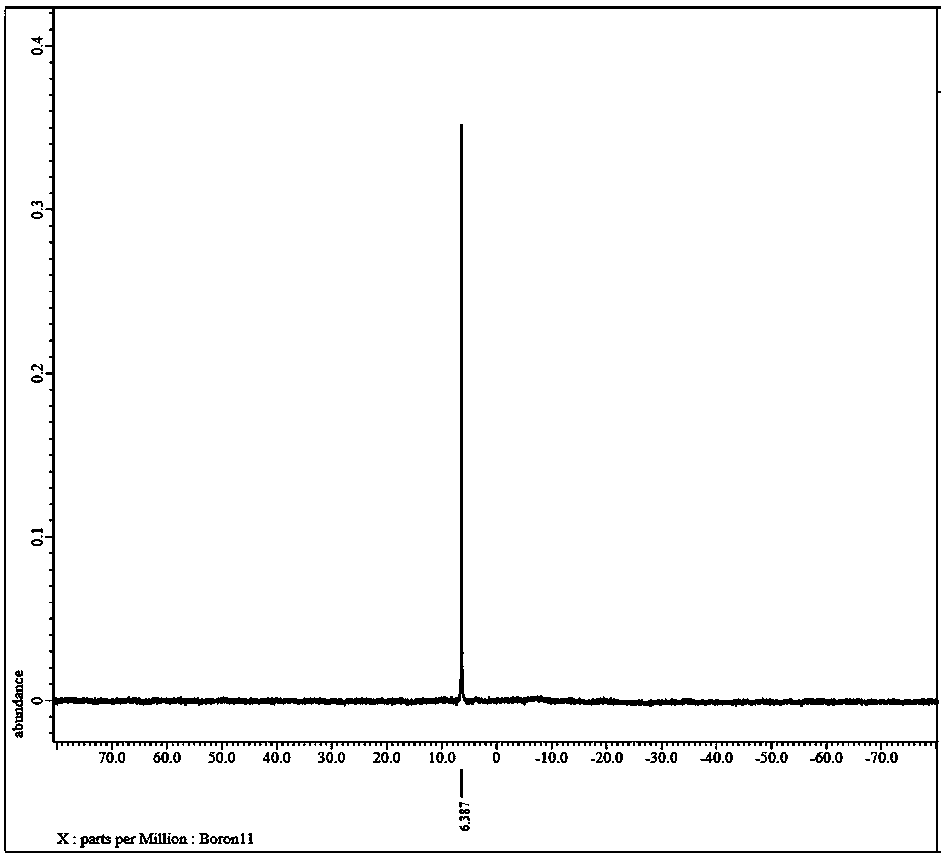
Preparation method of lithium bis(oxalato)borate
A bisoxalate lithium borate and oxalate technology is applied in chemical method analysis, chemical instruments and methods, chemical analysis by titration, etc. It can solve the problems of unfavorable industrial production, complicated reaction conditions, and uneven mixing, etc. Easy recovery, simple process, and the effect of increasing reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the method for preparing lithium bisoxalate borate mentioned in the present invention includes the following processes:
[0037] Dissolve 1058g of oxalic acid dihydrate and 248g of boric acid in 700g of water and heat and stir to dissolve to form solution A; 140g of lithium carbonate in 2000g of diethyl carbonate, stir and mix to form solution B; pump solution A and solution B uniformly at 50g / min Into the tubular reactor, keep the inside of the tubular reactor at 90°C and a pressure of -0.05kPa (gauge pressure); after the reaction, obtain a diethyl carbonate solution in which lithium bisoxalate borate is dissolved; Dry to obtain 745g crude LiBOB; dissolve the crude LiBOB in 5100g acetonitrile, heat to 45°C and stir for 2h; filter with suction to filter out the insoluble matter; make the filtrate distill out about 3026g acetonitrile at 45°C and pass through 4A molecular sieve, then transfer to crystallization Kettle, keep a certain stirring rate, start to coo...
Embodiment 2
[0038] Example 2, a method for preparing lithium bisoxalate borate mentioned in the present invention includes the following processes:
[0039] Dissolve 540g of anhydrous oxalic acid and 248g of boric acid in 1100g of water and heat and stir to dissolve to form solution A; 140g of lithium carbonate in 2000g of diethyl carbonate, stir and mix to form solution B; pump solution A and solution B uniformly at 50g / min Into the tubular reactor, keep the inside of the tubular reactor at 85°C and the pressure at -0.05kPa (gauge pressure); after the reaction, obtain a diethyl carbonate solution with lithium bisoxalate borate dissolved in it; Dry to obtain 709g crude LiBOB; dissolve the crude LiBOB in 3880g acetonitrile, heat to 40°C and stir for 2h; filter out the insoluble matter; distill out about 1552g acetonitrile from the filtrate at 45°C, pass through 3A molecular sieve, and transfer to the crystallization kettle to keep it constant The temperature is lowered at a rate of 7°C / min, a...
Embodiment 3
[0040] Embodiment 3, a method for preparing lithium bisoxalate borate mentioned in the present invention includes the following processes:
[0041] Dissolve 570g of lithium hydrogen oxalate and 248g of boric acid in 800g of water and heat and stir to form solution A; dissolve 134g of lithium hydroxide in 2500g of diethyl carbonate and stir and mix to form solution B; uniformize solution A and solution B at 50g / min pump Into the tubular reactor, keep the inside of the tubular reactor at 105°C and the pressure at -0.01kPa; after the reaction, obtain a solution of diethyl carbonate dissolved with lithium bisoxalate borate; evaporate and dry under reduced pressure to obtain LiBOB Crude product 712g; dissolve the crude product LiBOB in 4656g toluene, heat to 60℃ and stir for 3h; filter with suction to filter out the insoluble matter; make the filtrate distill out about 2328g toluene at 60℃, and then pass through 4A molecular sieve, transfer to the crystallization kettle and keep it con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com