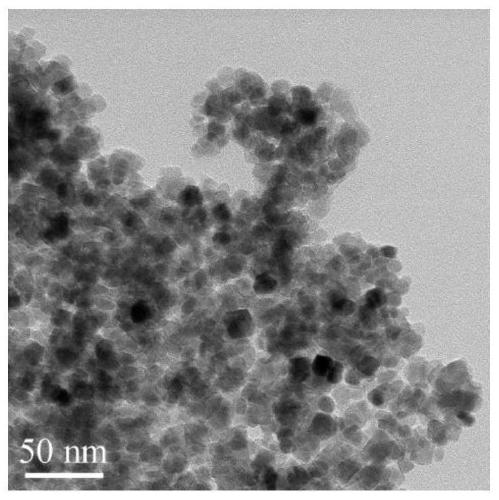
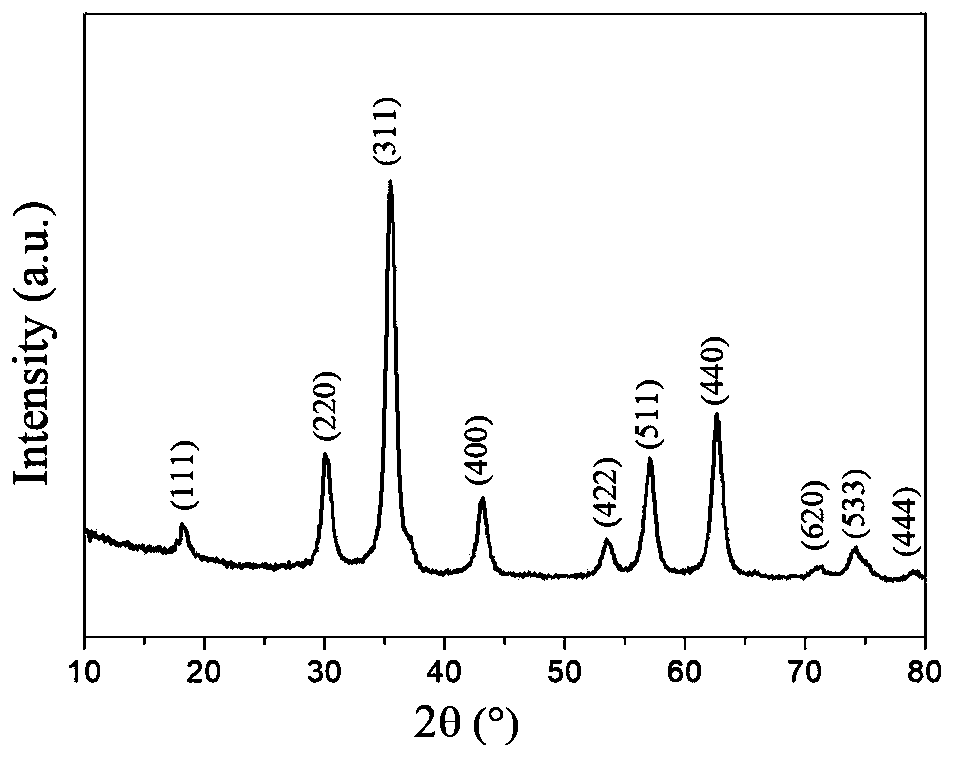
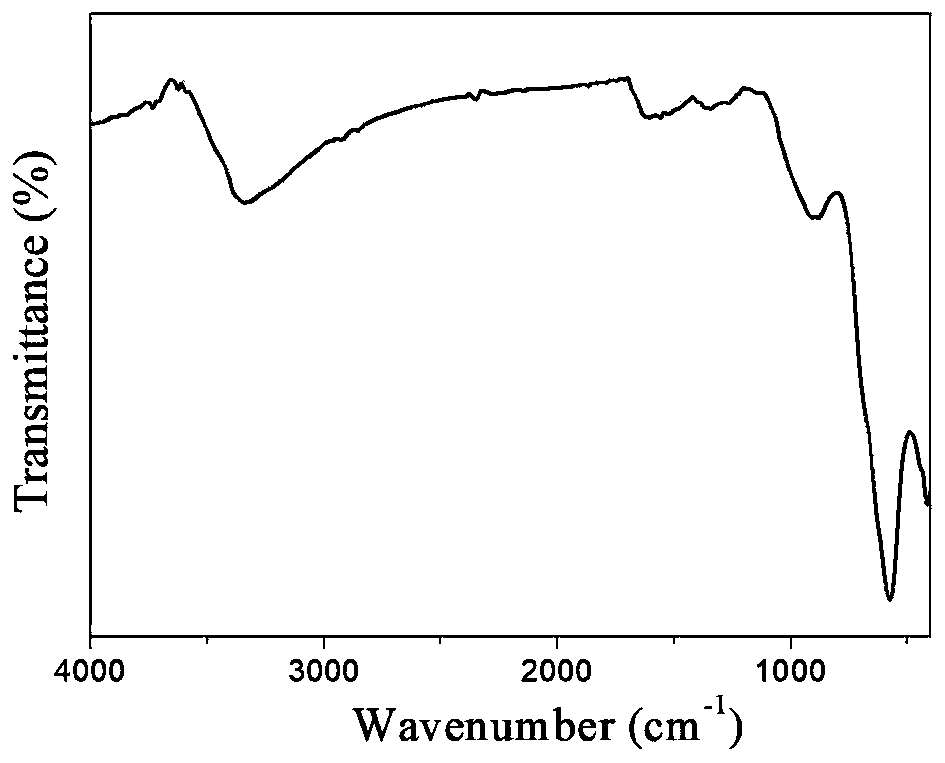
Method for decolorizing dye wastewater
A technology for dye wastewater and wastewater, applied in chemical instruments and methods, biochemical equipment and methods, water pollutants, etc., can solve problems such as easy deactivation, and achieve the effect of uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] A method for decolorizing dye wastewater, specifically: utilizing magnetic Cu(II) to chelate Fe 3 o 4 @C Nanoparticle-immobilized laccase decolorization of dye wastewater.
[0087] The preparation method of the magnetic copper (II) chelated ferric oxide@carbon nanoparticles comprises the following steps:
[0088] 1) Preparation of hydroxyl / carboxyl functionalized magnetic Fe 3 o 4 The operation process of @C nanoparticles is as follows:
[0089] 1-1) In a three-necked flask, dissolve 0.4mmol of ferrous chloride and 0.8mmol of ferric chloride in 50mL of water, and add 4mL of concentrated ammonia (28% concentration by mass) under stirring in a nitrogen atmosphere. Add ammonia water 3 times; first add 2mL concentrated ammonia water, react at 60°C for 20min, then add 1mL concentrated ammonia water at 60°C, react at 60°C for 20min, then add 1mL concentrated ammonia water, ℃ for 20 minutes, and finally heated up to 90 ℃ for 1 hour to prepare Fe 3 o 4 Nanoparticle suspe...
Embodiment 2
[0105] A method for the decolorization of dye wastewater, specifically: utilizing the magnetic Cu(II) chelated Fe prepared in Example 1 3 o 4 The method of @C nanoparticle immobilized laccase decolorization azo dye wastewater:
[0106] Prepare 0.1mol / L acetic acid buffer solution with pH=4.5; take 14mg ABTS and 50mg azo fluorescent pink and dissolve in 1000mL of the above-mentioned acetic acid buffer solution, the concentration of azo fluorescent pink is 50mg / L, as the azo fluorescent pink dye to be treated Wastewater; 4mg magnetic Cu(II) chelated Fe will be prepared by embodiment 1 3 o 4 The @C nanoparticle-immobilized laccase was dispersed into the above 8mL azo fluorescent pink dye wastewater, and reacted at 50°C for 1h under shaking conditions, and the decolorization rate of the azo fluorescent pink dye was measured by spectrophotometry.
[0107] Configure the acetic acid buffer solution of 0.1mol / L pH=4.5; Get 14mg ABTS and 20mg reactive red dye and be dissolved in 100...
Embodiment 3
[0110] A method for the decolorization of dye wastewater, specifically: utilizing the magnetic Cu(II) chelated Fe prepared in Example 1 3 o 4 The method of @C nanoparticle immobilized laccase decolorization anthraquinone dye wastewater:
[0111] Prepare 0.1mol / L acetic acid buffer solution with pH=4.5; take 14mg ABTS and 100mg anthraquinone dye Reactive Brilliant Blue-19 and dissolve them in 1000mL of the above acetic acid buffer solution to prepare 100mg / L anthraquinone dye solution; as the anthraquinone dye to be treated Wastewater; 4mg magnetic Cu(II) chelated Fe will be prepared by embodiment 1 3 o 4 @C nanoparticle-immobilized laccase was dispersed into the above 10ml anthraquinone dye solution, reacted at 50°C for 1 h under shaking conditions, and the decolorization rate of anthraquinone dye was measured by spectrophotometry.
[0112] Detection of the magnetic Cu(II) chelated Fe 3 o 4 The degradation rate of @C nanoparticle-immobilized laccase to reactive brilliant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com