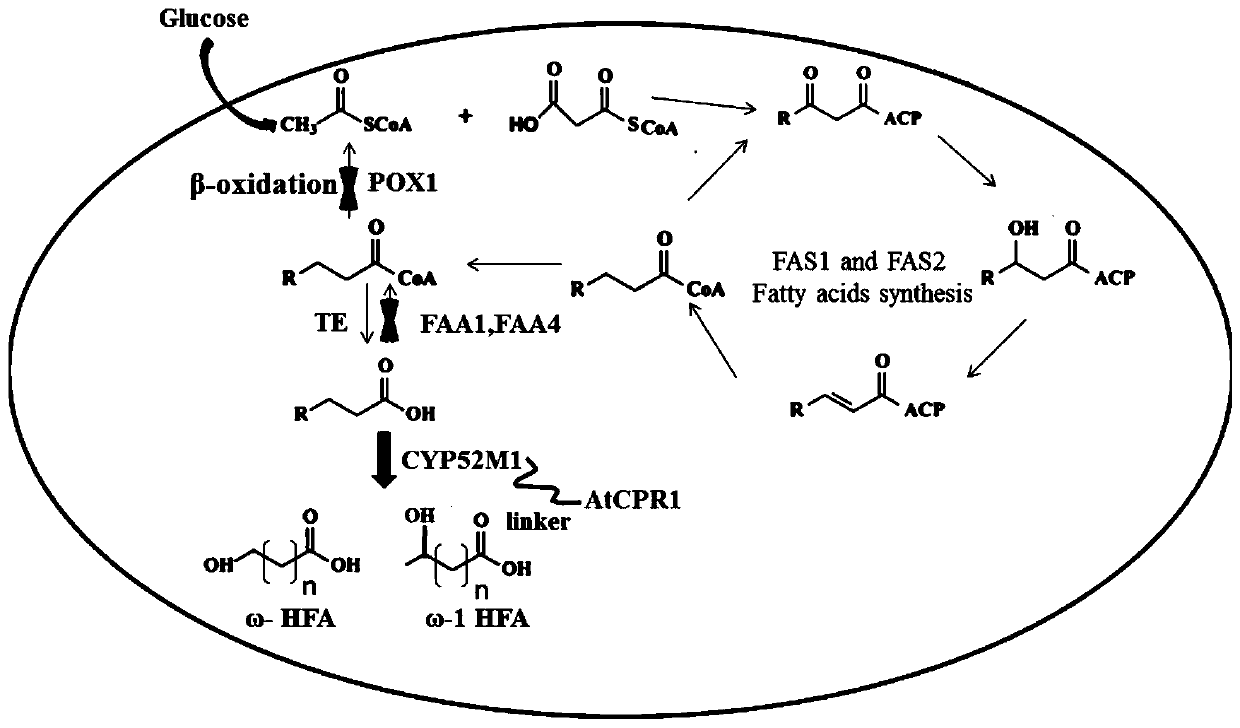
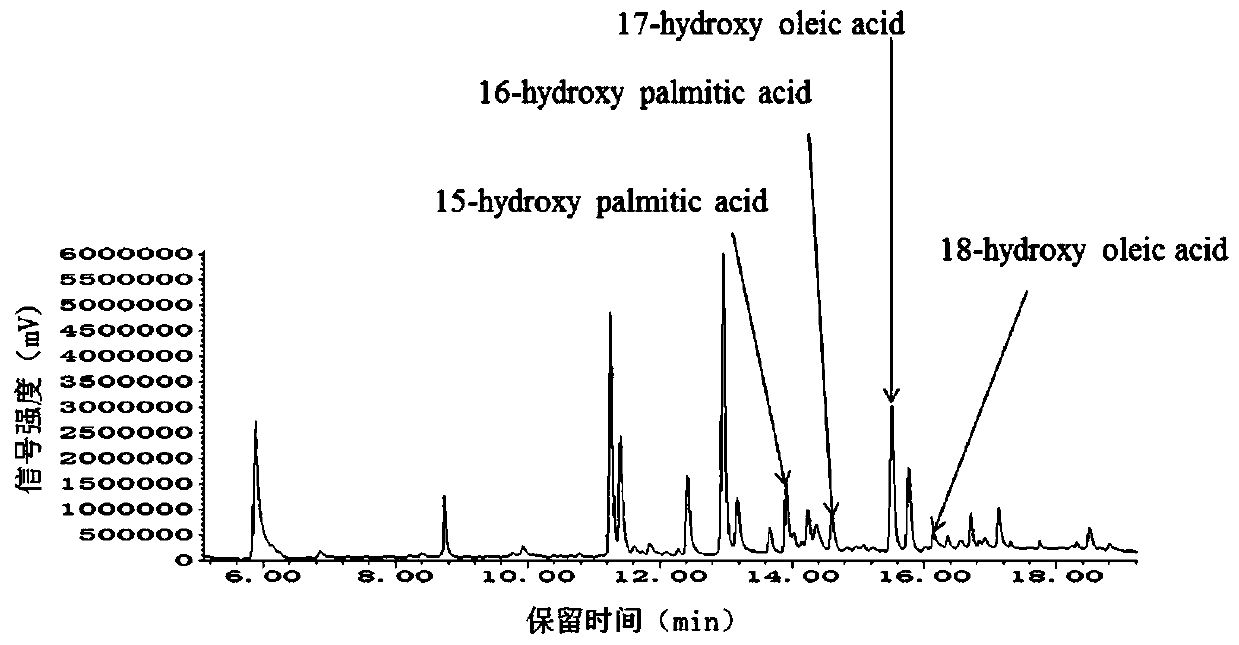
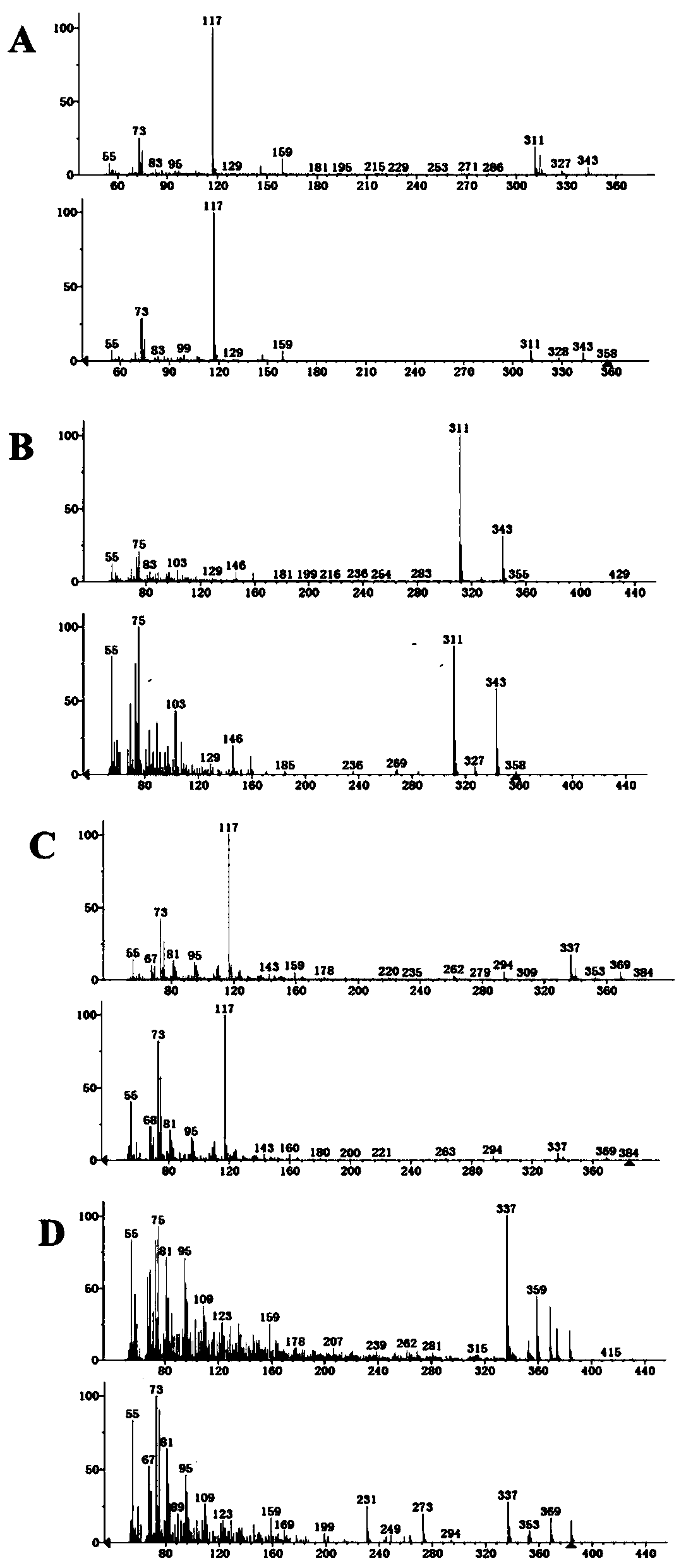
Recombinant saccharomyces cerevisiaes, construction methods of recombinant saccharomyces cerevisiaes and applications of recombinant saccharomyces cerevisiaes in production of hydroxy fatty acids
A technology for producing hydroxy fatty acid and Saccharomyces cerevisiae, which is applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 A kind of construction method of recombinant Saccharomyces cerevisiae (1) producing hydroxy fatty acid
[0037] module construction
[0038] According to the DNA sequences of the acyl-CoA oxidase encoding gene POX1, the acyl-CoA activating enzyme encoding gene FAA1 and the acyl-CoA activating enzyme encoding gene FAA4 provided on NCBI, design homology arms POX1A-up, POX1B-down, FAA1A-up , FAA1B-down, FAA4A-up, FAA4B-down. The templates were all from the genome of Saccharomyces cerevisiae BY4741; the selection marker gene his3 was from the plasmid Pxp320 (purchased from Addgene, Inc. www.addgene.org ).
[0039] Using the Saccharomyces cerevisiae BY4741 genome as a template,
[0040] With POX1A-F (SEQ ID NO.12),
[0041] HIS3-POX1A-R (SEQ ID NO.13) as primer
[0042] Amplified homology arm POX1A-up (SEQ ID NO.14)
[0043] With HIS3-POX1B-F (SEQ ID NO.15),
[0044] POX1B-R (SEQ ID NO.16) is a primer,
[0045] Amplified homology arm POX1B-down (SEQ ID ...
Embodiment 2
[0083] Embodiment 2, a kind of construction method of the recombinant Saccharomyces cerevisiae that produces hydroxy fatty acid (two)
[0084] Using the Saccharomyces cerevisiae BY4741 genome as a template, HOA-F (SEQ ID NO.38), PGK1p-HOA-R (SEQ ID NO.39) and HIS3-HOB-F (SEQ ID NO.40), HOB-R (SEQ ID NO.41) as primers to amplify the homology arms HOup (SEQ ID NO.42) and HOdown (SEQ ID NO.43); -R (SEQ ID NO.45) and CYP52M1-ADH1t-F (SEQ ID NO.46), TDH3p-ADH1t-R (SEQ ID NO.47) are primers to amplify the promoter P PGK1 (SEQ ID NO.7) and terminator T ADH1t (SEQ ID NO.8); using PGK1p-CYP52M1-F (SEQ ID NO.48) and ADH1t-CYP52M1-R (SEQ ID NO.49) as primers to amplify the cytochrome P450 oxidase gene CYP52M1 (SEQ ID NO .4); respectively with ADH1t-TDH3p-F (SEQ ID NO.50), SbCPR-TDH3p-R (SEQ ID NO.51) and SbCPR-TDH2t-F (SEQ ID NO.52), HIS3-TDH2t-R (SEQ ID NO.53) is a primer to amplify the promoter P TDH3 (SEQ ID NO.9) and terminator T TDH2t (SEQ ID NO.10); using TDH3p-SbCPR-F (SEQ ID...
Embodiment 3
[0086] Example 3, the construction method of recombinant Saccharomyces cerevisiae (three) producing hydroxy fatty acid
[0087] Using the Saccharomyces cerevisiae BY4741 genome as a template, HOA-F (SEQ ID NO.38), PGK1p-HOA-R (SEQ ID NO.39) and HIS3-HOB-F (SEQ ID NO.40), HOB-R (SEQ ID NO.41) as primers to amplify the homology arms HOup (SEQ ID NO.42) and HOdown (SEQ ID NO.43); -R (SEQ ID NO.45) and CYP52M1-ADH1t-F (SEQ ID NO.46), TDH3p-ADH1t-R (SEQ ID NO.47) are primers to amplify the promoter P PGK1 (SEQ ID NO.7) and terminator T ADH1t (SEQ ID NO.8); using PGK1p-CYP52M1-F (SEQ ID NO.48) and ADH1t-CYP52M1-R (SEQ ID NO.49) as primers to amplify the cytochrome P450 oxidase gene CYP52M1 (SEQ ID NO .4); respectively with ADH1t-TDH3p-F (SEQ ID NO.50), AtCPR1-TDH3p-R (SEQ ID NO.58) and AtCPR1-TDH2t-F (SEQ ID NO.59), HIS3-TDH2t-R (SEQID NO.53) is a primer to amplify the promoter P TDH3 (SEQ ID NO.9) and terminator T TDH2t (SEQ ID NO.10); with TDH3p-AtCPR1-F (SEQ ID NO.60) and TD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com