Preparation and application of MOF-guided nickel-cobalt bimetallic phosphide
A bimetallic and phosphide technology, applied in chemical/physical processes, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as high Tafel slope and large overpotential, and achieve excellent electrocatalytic performance and The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
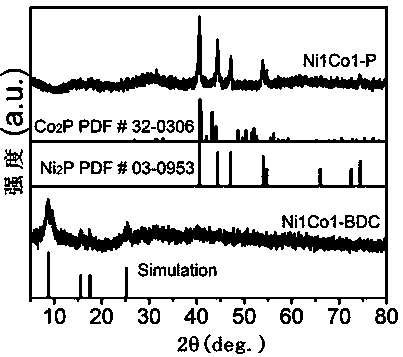
[0026] (1) Dissolve 0.75 mmol BDC in a mixed solution of 32 mL DMF, 2 mL ethanol and 2 mL deionized water at room temperature. After ultrasonication for 1 h and magnetic stirring for 0.5 h, 0.75 mmol NiCl 2 •6H 2 O and 0.75 mmol CoCl 2 •6H 2 O was added to the above mixed solution, then 0.8 mL of ETA was rapidly injected, the solution was magnetically stirred for 5 min to form a uniform suspension, and further sonicated continuously for 8 h under sealed conditions, and finally washed with DMF and ethanol alternately 3 times and collected by centrifugation. Ni1Co1-BDC was obtained by vacuum drying at 60℃;
[0027] (2) Combine 50mg Ni1Co1-BDC and 250mg NaH 2 PO 2 •H 2 O is placed in two positions of the porcelain boat, Ni1Co1-BDC is placed upstream of the porcelain boat, NaH 2 PO 2 •H 2 O was placed downstream of the porcelain boat, and then the porcelain boat was put into a tube furnace, calcined in an Ar atmosphere, and kept at 300 °C for 2 h. After the calcination is ...
Embodiment 2
[0030] (1) Same as Example 1;
[0031] (2) Combine 50mg Ni1Co1-BDC and 250mg NaH 2 PO 2 •H 2 O is placed in two positions of the porcelain boat, Ni1Co1-BDC is placed upstream of the porcelain boat, NaH 2 PO 2 •H 2 O was placed downstream of the porcelain boat, and then the porcelain boat was placed in a tube furnace, calcined in an Ar atmosphere, and kept at 280 °C for 2.5 h. After the calcination is completed, the temperature is cooled to room temperature, washed three times with deionized water, collected by centrifugation, and dried under vacuum at 60 °C to obtain Ni1Co1-P;
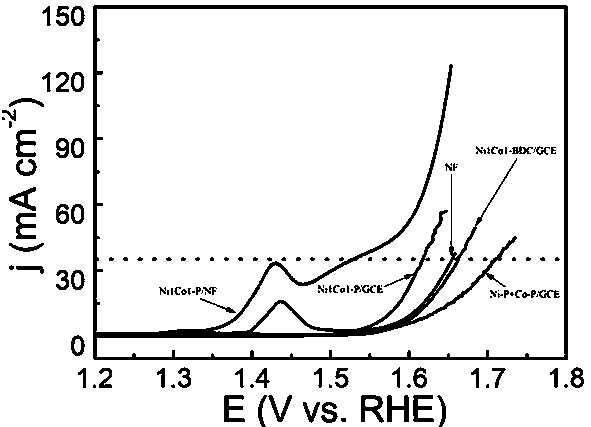
[0032] (3) Electrochemical performance test of Ni1Co1-P / NF: at a current density of 40 mA / cm 2 Overpotential is 274mV, Tafel is 80mV dec -1 .
Embodiment 3
[0034] (1) Same as Example 1;
[0035] (2) Combine 50mg Ni1Co1-BDC and 250mg NaH 2 PO 2 •H 2 O is placed in two positions of the porcelain boat, Ni1Co1-BDC is placed upstream of the porcelain boat, NaH 2 PO 2 •H 2 O was placed downstream of the porcelain boat, and then the porcelain boat was placed in a tube furnace, calcined in an Ar atmosphere, and kept at 320 °C for 1.5 h. After the calcination is completed, the temperature is cooled to room temperature, washed three times with deionized water, collected by centrifugation, and dried under vacuum at 60 °C to obtain Ni1Co1-P;
[0036] (3) Electrochemical performance test of Ni1Co1-P / NF: at a current density of 40 mA / cm 2 Overpotential is 284mV, Tafel is 85mV dec -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com