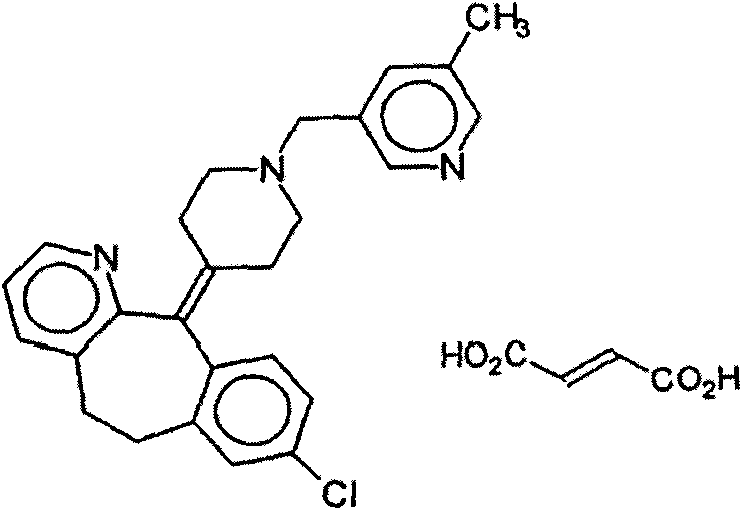
Oral liquid preparation of rupatadine fumarate and preparation method of oral liquid preparation
A technology for rupatadine fumarate and liquid preparations, which is applied in the field of rupatadine fumarate oral liquid preparations and its preparation, and can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1-preparation of oral liquid preparation of the present invention
[0020] prescription:
[0021]
[0022]
[0023] Preparation method:
[0024] 1. Weigh the prescribed amount of rupatadine fumarate, polyethylene glycol 600, xylitol, sodium saccharin dihydrate, quinoline yellow;
[0025] 2. Add the above raw and auxiliary materials into about 80ml of purified water, and stir until it dissolves;
[0026] 3. Prepare citric acid-sodium citrate buffer solution with pH 5.0;
[0027] 4. Adjust with pH 5.0 citric acid-sodium citrate buffer solution to adjust the pH value of the raw material solution to 5.0;
[0028] 5. Dilute to 100ml with purified water;
Embodiment 2
[0029] Embodiment 2-preparation of oral liquid preparation of the present invention
[0030] prescription:
[0031]
[0032] Preparation method:
[0033] 1. Weigh the prescribed amount of rupatadine fumarate, povidone K25, xylitol, sodium saccharin dihydrate, quinoline yellow, sodium bisulfite, methylparaben;
[0034] 2. Add the above raw and auxiliary materials into about 80ml of purified water, and stir until it dissolves;
[0035] 3. Prepare a citric acid-sodium citrate buffer solution with a pH of 4.8;
[0036] 4. Adjust with pH 4.8 citric acid-sodium citrate buffer solution to adjust the pH value of the raw material solution to 4.8;
[0037] 5. Dilute to 100ml with purified water;
Embodiment 3
[0038] Embodiment 3-preparation of oral liquid preparation of the present invention
[0039] prescription:
[0040]
[0041]
[0042] Preparation method:
[0043] 1. Weigh the prescribed amount of rupatadine fumarate, povidone K25, xylitol, sodium saccharin dihydrate, orange flavor, quinoline yellow, sodium bisulfite, methylparaben;
[0044] 2. Add the above raw and auxiliary materials into about 80ml of purified water, and stir until it dissolves;
[0045] 3. Prepare citric acid-sodium citrate buffer solution with pH 5.0;
[0046] 4. Adjust with pH 5.0 citric acid-sodium citrate buffer solution to adjust the pH value of the raw material solution to 5.0;
[0047] 5. Dilute to 100ml with purified water;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com