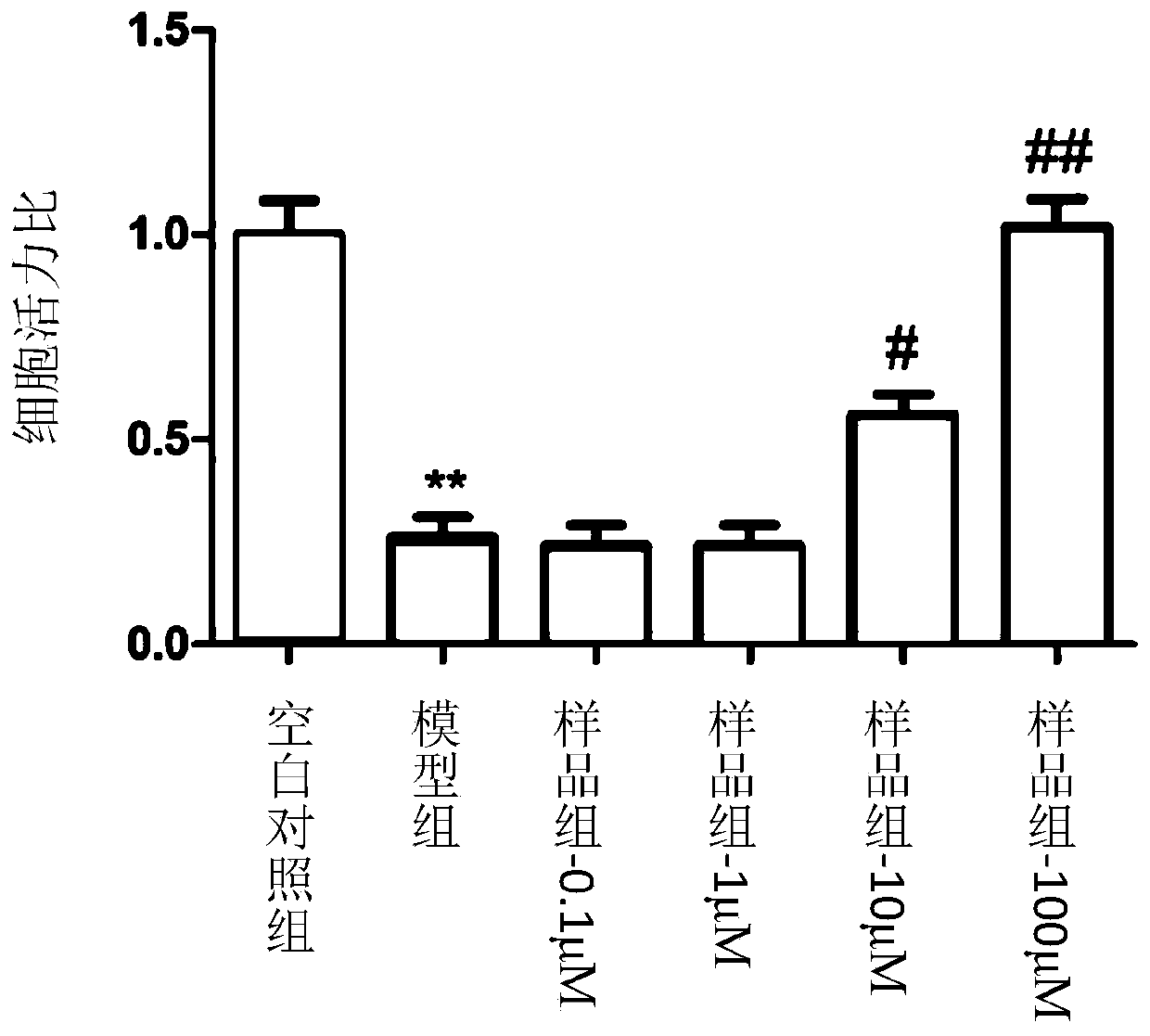
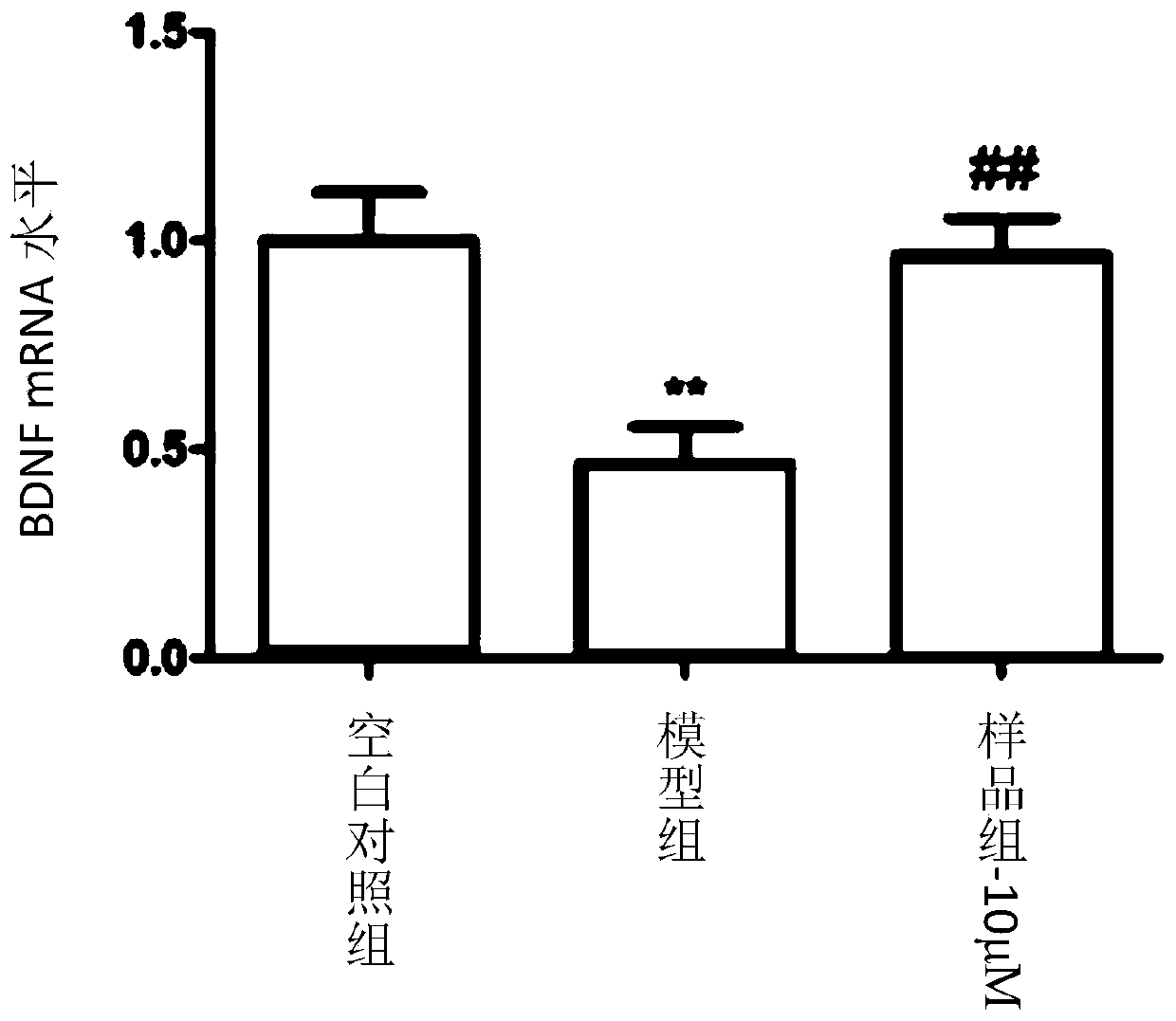
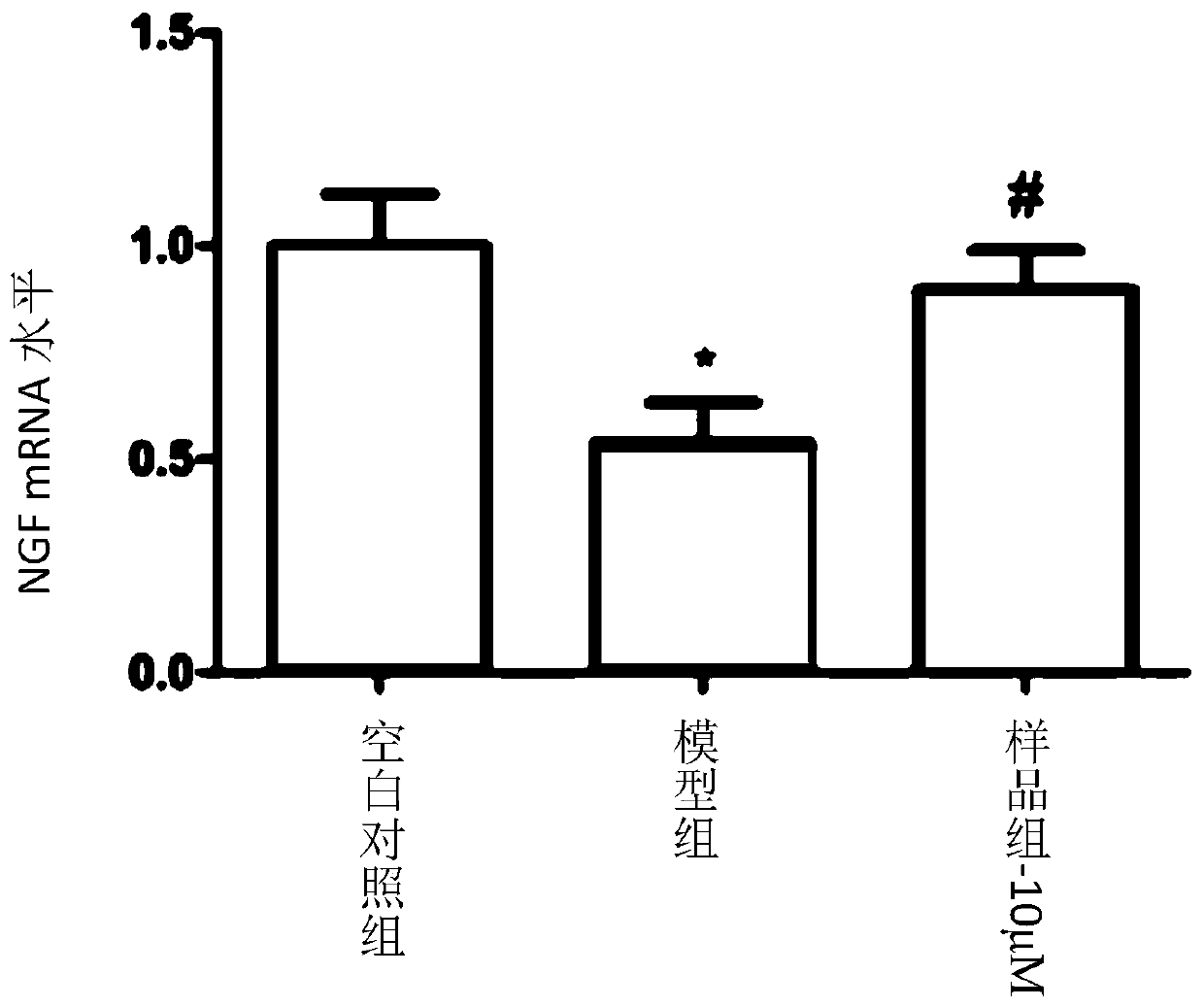
Coumarin dimer compound, pharmaceutical composition, and preparation method and application of the coumarin dimer compound
A technology of coumarins and compounds, which is applied in the field of natural medicinal chemistry, can solve the problem of lack of therapeutic drugs, and achieve the effects of improving expression and nerve cell damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0092] The characterization of embodiment 2 formula I compound
[0093]
[0094] Ultraviolet spectrum (UV) shows strong absorption at 297nm and 326nm, indicating that the compound is a coumarin compound. High resolution mass spectrometry gave the molecular formula C 28 h 26 o 7 , [M+Na] + :497.1566(calcd 497.1576), see Figure 11 .
[0095] Compound (I) in 1 H-NMR spectrum (see Figure 12 ) low-field region showing characteristic hydrogen signals on a pair of coumarin backbones [δ H 6.20(1H, d, J=9.4Hz, H-3), 7.83(1H, d, J=9.4Hz, H-4)], and a set of hydrogen signals of the ABX system [δ H 7.54(1H,d,J=8.6Hz,H-5),7.09(1H,dd,J=8.6,2.3Hz,H-6),7.32(1H,d,J=2.3Hz,H-8)] , and a pair of coupled oxygen-proton signals [δ H 6.24 (1H, d, J=2.3Hz, H-9), 4.69 (1H,d, J=2.9Hz, H-10)]. At the same time, in 13 The C-NMR spectrum shows a total of 28 carbon signals, which are respectively assigned to two coumarin units, as shown in Table 1.
[0096] Compound (I) in 1 H- 1 In the...
preparation experiment example 1
[0100] Preparation experiment example 1 (preparation of injection)
[0101] Take 1000 mg of the compound represented by formula I obtained in Example 1 above, add 1000 ml of water for injection, adjust the pH value to 7 with sodium carbonate, stir to dissolve, sterilize and filter, pot, and circulate steam at 100 ° C for 15 minutes Sterilize, and make each injection containing 2mg / 2ml of the compound of formula I for injection.
preparation experiment example 2
[0102] Preparation experiment example 2 (preparation of capsules)
[0103]
[0104] Weigh the compound shown in formula I obtained in Example 1 according to the weight of the above formula, microcrystalline cellulose, sodium carboxymethyl starch, sodium lauryl sulfate, mix, adopt the roller compaction method to carry out dry granulation, and then mix with hard Magnesium fatty acid is mixed, filled into 3# hollow capsules, and made into capsules containing formula I compound 100mg / grain for oral use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com