Preparation method of high-saturation magnetization superparamagnetic porous ferrite microspheres
A superparamagnetic and porous microsphere technology, applied in chemical instruments and methods, iron oxide/iron hydroxide, diamagnetic/paramagnetic materials, etc. The magnetic properties of microspheres and nanocrystalline assemblies have a single structure, etc., to achieve the effects of green and non-toxic raw materials, good magnetic response performance, and high saturation magnetization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
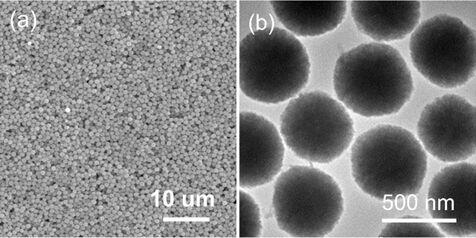
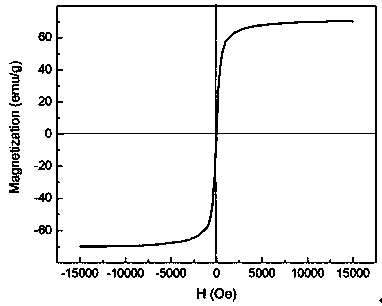
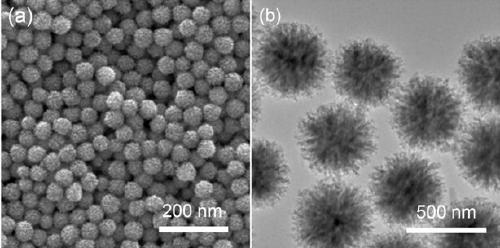
Image
Examples
Embodiment 1
[0035] Embodiment 1 A kind of preparation method of highly saturated magnetized superparamagnetic ferrite porous microsphere
[0036] A high saturation magnetization superparamagnetic Fe 3 o 4 The preparation of porous microspheres, the preparation process comprises the following steps:
[0037] 1. Superparamagnetic Fe 3 o 4 Synthesis of microspheres
[0038] (1) Preparation of precursor solution
[0039] At room temperature, 15 mmol ferric chloride hexahydrate and 150 mmol anhydrous sodium acetate were dissolved in 60 mL of ethylene glycol, and the ethylene glycol solution of ferric chloride hexahydrate was slowly added to the ethyl alcohol solution of anhydrous sodium acetate. In the diol solution, stir for 30min under magnetic stirring, mix evenly, and then add polyacrylic acid (M w 1800) 0.4 g was added to the above mixed solution, and the stirring was continued for 2 h to obtain a uniform yellow precursor solution.
[0040] (2) Heating reaction
[0041]Transfer th...
Embodiment 2
[0060] Example 2 A preparation method of highly saturated magnetized superparamagnetic ferrite porous microspheres
[0061] A High Saturation Magnetization Superparamagnetic Porous MnFe 2 o 4 The preparation of microspheres, the preparation process comprises the following steps:
[0062] 1. Superparamagnetic MnFe 2 o 4 Synthesis of microspheres
[0063] (1) Preparation of precursor solution
[0064] At room temperature, 5 mmol MnCl 2 , 10mmolFeCl 3 ·6H 2 O was dissolved in 60mL of ethylene glycol, weighed 150mmol of anhydrous sodium acetate and dissolved in 60mL of ethylene glycol, slowly added the ethylene glycol solution containing cobalt and iron into the ethylene glycol solution of anhydrous sodium acetate, and Stir for about half an hour under stirring, mix evenly, and then add polyacrylic acid (M w 1820) 0.43g was added to the above mixed solution, and the stirring was continued for 2h to obtain a uniform yellow precursor solution.
[0065] (2) Heating reaction...
Embodiment 3
[0080] Example 3 A preparation method of highly saturated magnetized superparamagnetic ferrite porous microspheres
[0081] A High Saturation Magnetization Superparamagnetic Porous ZnFe 2 o 4 The preparation of microspheres, the preparation process comprises the following steps:
[0082] 1. Superparamagnetic ZnFe 2 o 4 Synthesis of microspheres
[0083] (1) Preparation of precursor solution
[0084] At room temperature, 6 mmol ZnCl 2 , 12mmolFeCl 3 ·6H 2 O is dissolved in 70mL ethylene glycol, weighs 180mmol anhydrous sodium acetate and dissolves it in 70mL ethylene glycol, slowly adds the ethylene glycol solution containing cobalt and iron into the ethylene glycol solution of anhydrous sodium acetate, and Stir for about half an hour under stirring, mix evenly, and then add polyacrylic acid (M w 1850) 0.47 g was added to the above mixed solution, and the stirring was continued for 3 h to obtain a uniform yellow precursor solution.
[0085] (2) Heating reaction
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com