Antifungal composition and application thereof
A composition and antifungal technology, applied in the field of medicine, can solve the problems of poor treatment effect, greasy nails, easy to be washed off and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1. The synergistic antifungal effect of terbinafine hydrochloride and berberine hydrochloride
[0027] By comparing and judging the MIC values of terbinafine hydrochloride and berberine hydrochloride and their combined use, the advantages of the combination of the two drugs in vitro are illustrated. This study was carried out with reference to the protocol and related literature formulated by the National Clinical Laboratory Standardization Institute (CLSI).
[0028] Experimental method: (1) Configuration of drug concentration: single-drug terbinafine hydrochloride TBH and berberine hydrochloride BRH were prepared with dimethyl sulfoxide to prepare mother solutions with concentrations of 50000 μg / mL and 10000 μg / mL, and then used RPMI1640 liquid medium was diluted 100-fold and 20-fold with the above mother liquid respectively to a maximum concentration of 500 μg / mL, and then diluted with RPMI1640 liquid medium to obtain 20 concentrations, and the final conc...
Embodiment 2
[0032] Embodiment 2. Treat the coating agent of onychomycosis infection disease
[0033] (1) The single drug composition (containing urea) of this embodiment includes the following raw materials by mass percentage:
[0034] Terbinafine Hydrochloride 1%
[0035] Eudragit ® RLPO 20%
[0036] Ethanol 55%
[0037] Urea 10%
[0038] water 14%
[0039] The preparation method of this embodiment composition:
[0040] A, according to the formula ratio, at room temperature, terbinafine hydrochloride and ethanol are mixed to form a transparent and clear solution;
[0041] B. Add RLPO to the solution described in A according to the formula ratio;
[0042] C. According to the formula ratio, at room temperature, urea is mixed with water and ultrasonicated at 25°C to form a uniform and clear urea solution, which is added to the solution described in step B to obtain C solution;
[0043] D. Stir the C solution continuously until the RLPO is dissolved to form a uniform, stable, transp...
Embodiment 4
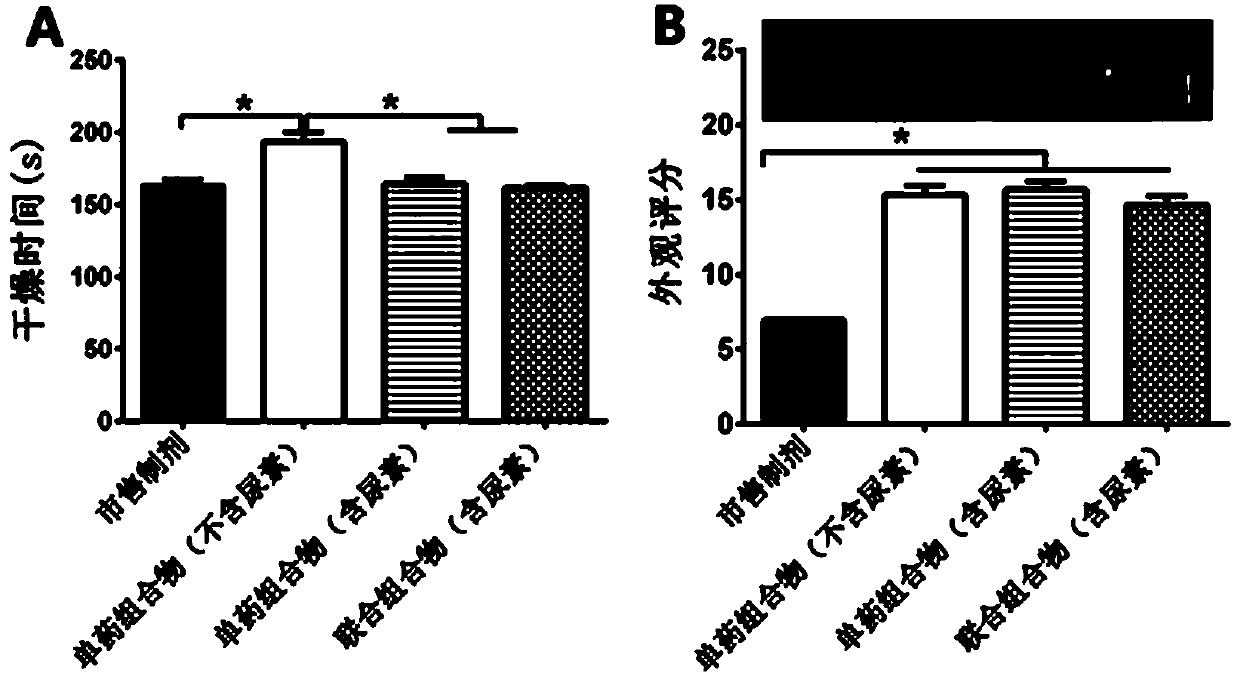
[0086] Embodiment 4. the evaluation of the coating agent for the treatment of onychomycosis infection
[0087] 1. Film forming (drying time and appearance score) experiment
[0088] The combination composition (containing urea), single drug composition (containing urea), single drug composition (excluding urea) and the commercially available preparation (Lamisil Pedisan ® once) for film-forming experiments.
[0089] Test product: 0.2ml of the combined composition of the present invention (containing urea); terbinafine hydrochloride: 0.5%, berberine hydrochloride: 0.5%, and the properties are clear and transparent liquid; 0.2ml of the single drug composition of the present invention (containing urea ); Terbinafine hydrochloride: 1%, the properties are clear and transparent liquid; reference substance: 0.2ml composition (excluding urea), terbinafine hydrochloride: 1%, the properties are clear and transparent liquid; 0.2ml commercially available preparation (Lamisil Pedisan ®...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com