D-A type excited state proton transfer high-efficiency fluorescent material, and preparation method and application thereof
A technology of proton transfer and fluorescent materials, applied in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems of separation and low fluorescence quantum efficiency of D-A molecules, achieve easy purification, and improve exciton utilization. and external quantum efficiency, synthesizing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
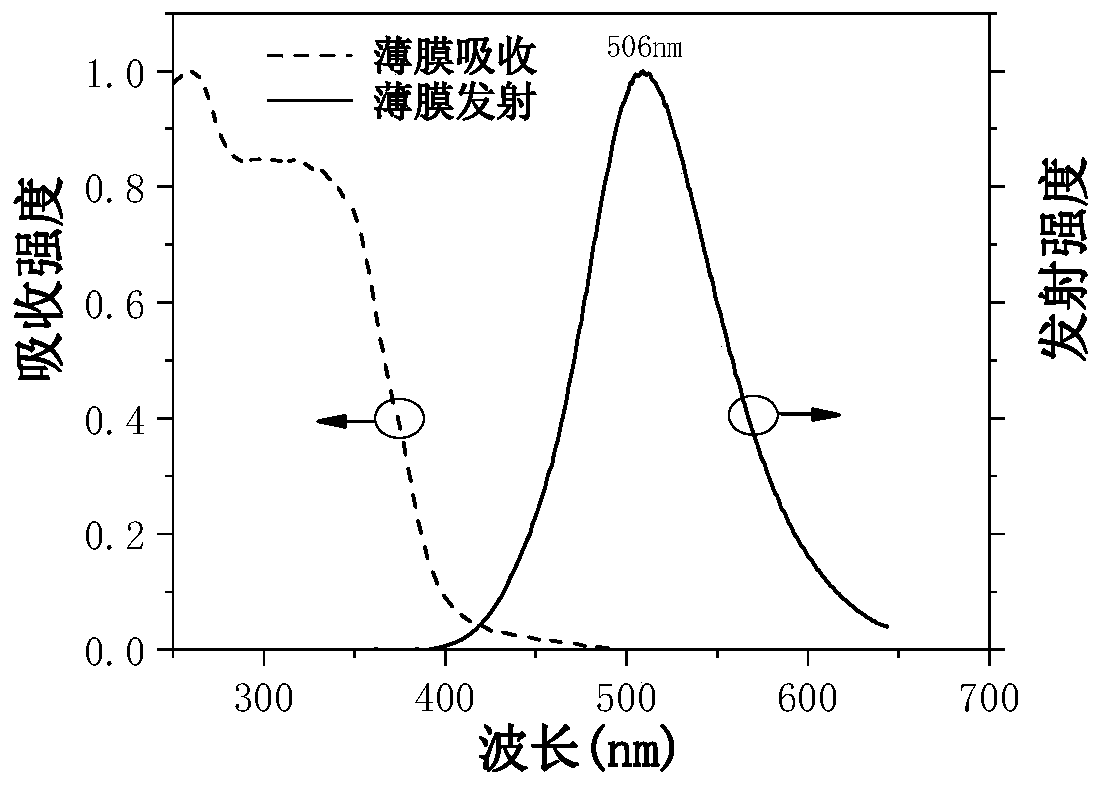
Image
Examples
Embodiment 1
[0027] The structural formula of the organic small molecule fluorescent material of the D-A structure of the present embodiment is as follows:
[0028]
[0029] The preparation method of the organic small molecule fluorescent material 5-TPA-PPI-OH of the D-A structure of the present embodiment comprises the following steps:
[0030] Step 1: the synthesis (M2) of triphenylamine boroester, the reaction equation is as follows:
[0031]
[0032] Step 1-1 (synthesis of M1): Put triphenylamine (10 g, 40.8 mmol) and 50 ml of chloroform into a 250 ml round bottom flask, and wrap the outside of the flask with aluminum foil to avoid light. N-bromosuccinimide (NBS, 7.5 g, 42 mmol, 1.02 equiv) was added to the flask in several portions. The mixture was stirred and reacted at room temperature in the dark for 24 hours. After the reaction, the reaction solution was washed three times with deionized water and extracted with dichloromethane. The extract was dried with an appropriate a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com