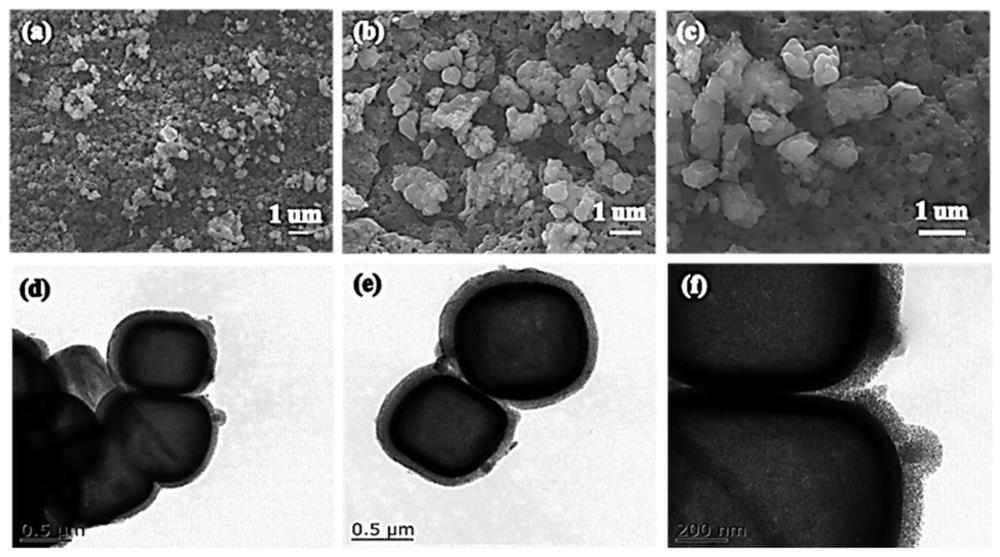
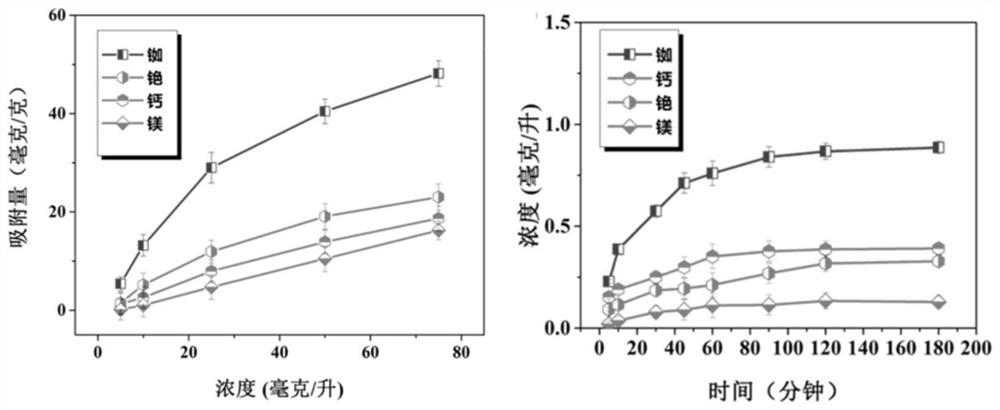
A kind of preparation method and application of ion separation membrane
An ion separation, polyvinylidene fluoride technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] S1. Preparation of mesoporous silicon:
[0029] Soak the prepared 0.2 g of ferric oxide in the mixture A composed of 80 mL of deionized water, 300 mL of ethanol and 12 mL of ammonia water, ultrasonically uniform, and stir rapidly for 4 h. During the stirring process, 20 mL of ethanol and 1.2 mL of tetraethyl orthosilicate mixed solution B was added dropwise to the above solution, sealed, centrifuged, washed, and dried to obtain ferric oxide coated with a layer of silicon balls, after 0.1 g silicon-coated Fe2O3 and 0.2 g cetyltrimethylammonium bromide were soaked in a mixture A consisting of 50 mL deionized water, 65 mL ethanol and 1 mL ammonia water, ultrasonically uniformed, and stirred rapidly for 8 h. During the stirring process, the mixed solution B composed of 20 mL ethanol and 0.13 mL ethyl orthosilicate was added dropwise to the above solution, sealed, centrifuged, washed, dried, and calcined at 550 °C for 4 h to obtain mesoporous silicon .
[0030] S2. Prepara...
Embodiment 2
[0041] S1. Preparation of mesoporous silicon:
[0042] First, soak the prepared 0.2 g of ferric oxide in the mixture A composed of 80 mL of deionized water, 300 mL of ethanol and 12 mL of ammonia water, ultrasonically uniform, and stir rapidly for 6 h. During the stirring process, 20 Mixed solution B consisting of 1 mL ethanol and 1.2 mL ethyl tetrasilicate was added dropwise to the above solution, sealed, centrifuged, washed, and dried to obtain ferric oxide coated with a layer of silicon balls, and 0.1 g of Diferric oxide and 0.2 g cetyltrimethylammonium bromide after silicon were soaked in a mixture A consisting of 50 mL deionized water, 65 mL ethanol and 1 mL ammonia water, ultrasonically uniformed, and stirred rapidly for 6 h. During the stirring process, the mixed solution B composed of 20 mL ethanol and 0.13 mL tetraethyl orthosilicate was added dropwise to the above solution, sealed, centrifuged, washed, dried, and calcined at 450 ° C for 6 hours to obtain mesoporous s...
Embodiment 3
[0052] S1. Preparation of mesoporous silicon:
[0053] Soak the prepared 0.2 g of ferric oxide in the mixture A composed of 80 mL of deionized water, 300 mL of ethanol and 12 mL of ammonia water, ultrasonically uniform, and stir rapidly for 4 h. During the stirring process, 20 mL of ethanol and 1.2 mL of tetraethyl orthosilicate mixed solution B was added dropwise to the above solution, sealed, centrifuged, washed, and dried to obtain ferric oxide coated with a layer of silicon balls, after 0.1 g silicon-coated Soak Fe2O3 and 0.2 g cetyltrimethylammonium bromide in a mixture A consisting of 50 mL deionized water, 65 mL ethanol and 1 mL ammonia water, ultrasonically homogenize, and stir rapidly for 4 h. During the stirring process, the mixed solution B composed of 20 mL ethanol and 0.13 mL ethyl orthosilicate was added dropwise to the above solution, sealed, centrifuged, washed, dried, and calcined at 650 °C for 3 hours to obtain mesoporous silicon.
[0054] S2, the preparatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com