Synthesis and purification method of antiviral drug key intermediate
An antiviral drug and purification method technology, applied in the field of drug synthesis, can solve the problems of low total product yield, uncontrollable factors in the process, long reaction route, etc., to achieve controllable process, reduce reaction steps, and facilitate large-scale production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
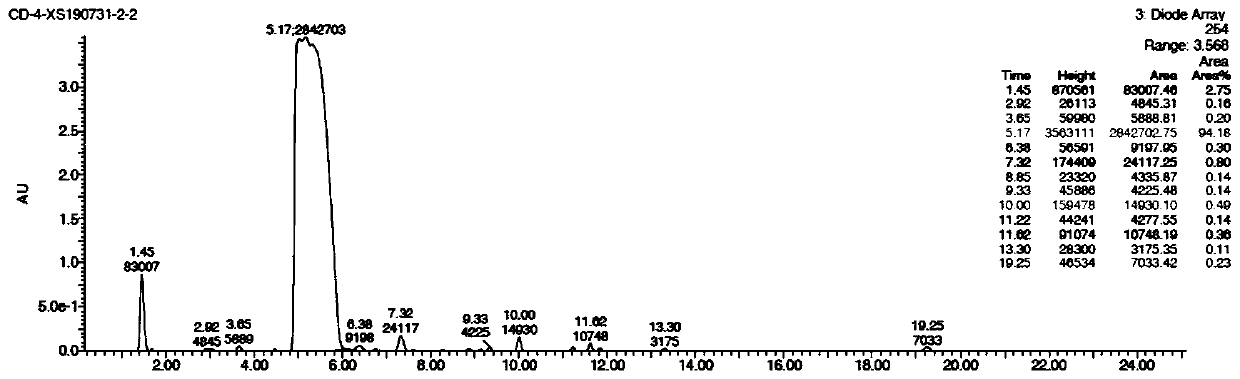
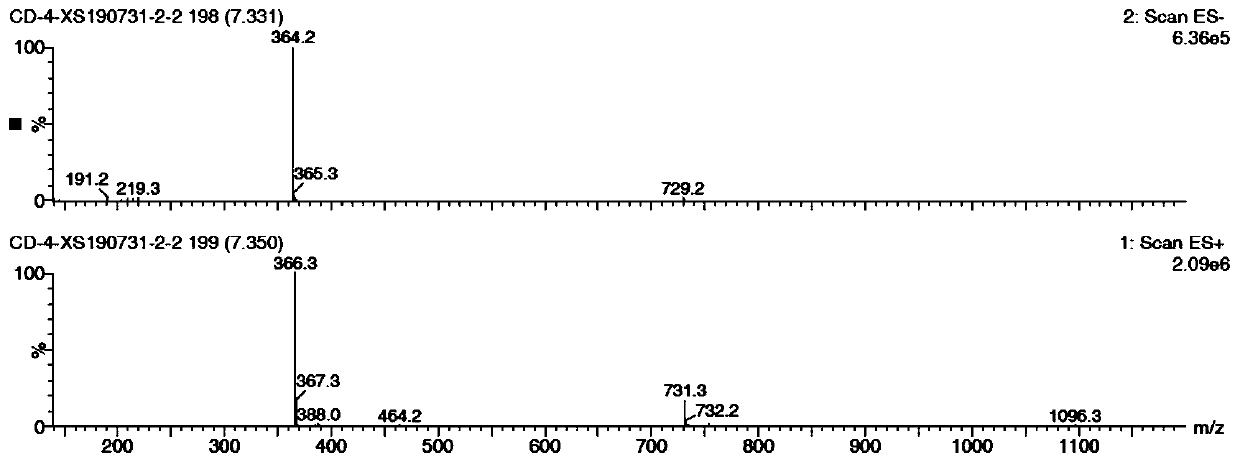
[0022] (s)-N4-benzoyl-N 1 Synthesis of -[(2-diethylphosphomethoxy-3-trityloxy)propyl]cytosine
[0023] 50ml of anhydrous DMF and N4-benzamide cytosine (5.00g, 23.23mmol) were heated to 100°C, 60% NaH (0.23g, 5.81mmol) was added in one portion, and the slurry was stirred for 15min. Then S-trityloxymethyloxirane (8.82 g, 27.88 mmol) was added, and the temperature was raised to 110° C. for 3 h. Immediately after the reaction, NaH (1.86g, 46.46mmol) and diethyl p-toluenesulfonyloxymethylphosphate (11.23g, 34.85mmol) were added, and reacted overnight at 100°C. The reaction mixture was quenched with water, ethyl acetate was added to separate the organic phase, the organic phase was concentrated to dryness, and recrystallized with a mixed solvent of ethyl acetate and n-hexane to obtain about 10.8 g of off-white powdery solid (yield 68.2%).
Embodiment 2
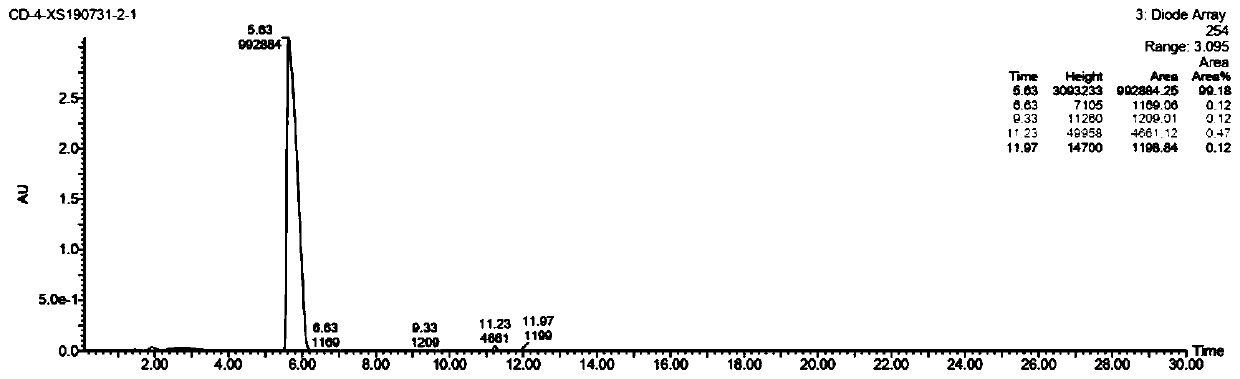
[0025] The key intermediate of cidofovir (s)-N4-benzoyl-N 1 Synthesis of -[(2-phosphomethoxy-3-hydroxy)propyl]cytosine
[0026] (s)-N4-benzoyl-N that embodiment 1 obtains 1 -[(2-Diethylphosphomethoxy-3-tritylmethoxy)propyl]cytosine (4.00g, 5.87mmol) was dissolved in 40ml of dichloromethane, 16ml of 40% hydrobromic acid was added, at room temperature After reacting overnight, TLC monitored the disappearance of the raw material point, stopped the reaction, separated the liquid, adjusted the pH of the aqueous phase to neutral, and precipitated a white solid, which was filtered to obtain 1.88 g of the crude product of the target (yield 83.6%).
Embodiment 3
[0028] The key intermediate of cidofovir (s)-N4-benzoyl-N 1 Synthesis of -[(2-phosphomethoxy-3-hydroxy)propyl]cytosine
[0029] Dissolve 4.00g of CD-1 in 40ml of tetrahydrofuran, add 16ml of 40% hydrobromic acid, react at room temperature overnight, TLC monitors that the raw material point disappears, stop the reaction, evaporate the organic solvent under reduced pressure, and adjust the pH of the remaining water phase to neutral to precipitate a white solid , and filtered to obtain about 1.80 g of the target crude product (yield 87.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com