Preparation method of baloxavir intermediate
An intermediate and preparation process technology, which is applied in the field of preparation of baloxavir intermediates, can solve the problems of unfavorable industrial production, low three-step yield, long reaction route, etc., achieve stable industrial production and preparation, and avoid chiral disassembly points, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068]
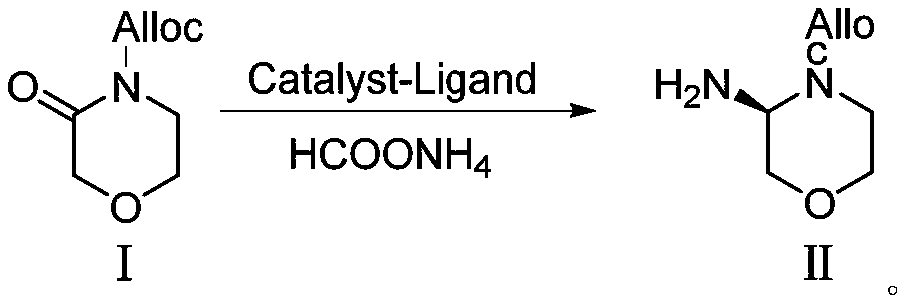
[0069] 3-Morpholinone (10.1 g, 0.1 mol) and THF (100 mL) were added to the reaction flask, cooled to -78°C, and n-butyllithium solution (68 mL, 0.11 mol) was added dropwise under nitrogen. After the dropwise addition was completed, it was stirred at -78°C for 2h, and a THF (60mL) solution of allyl chloroformate (12g, 0.1mol) was added dropwise to the reaction solution, and after the dropwise addition was completed, it was stirred for 2h, then quenched by adding a saturated ammonium chloride solution. , after warming to room temperature, extracted with ethyl acetate, washed with brine, and concentrated under reduced pressure to obtain 14.8 g of compound I (yield 80%). 1 H-NMR (CDCl 3 )3.63(t,2H),3.72(t,2H),4.26(s,2H),4.58(d,2H),5.35(d,1H),5.45(d,1H),5.90-6.02(m,1H ).
Embodiment 2
[0071]
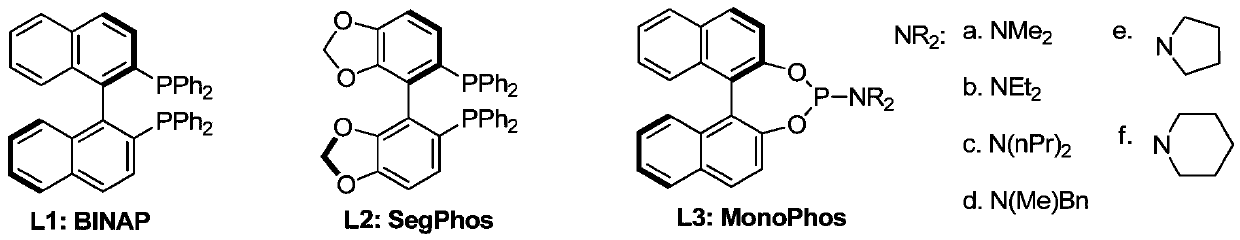
[0072] Under nitrogen, the [Ir(COD)Cl] 2 (0.2mmol) and phosphine ligand L3f (0.4mmol) were dissolved in DCM (10mL), stirred at room temperature for 20min to make it completely complexed, compound Ⅰ (14.8g, 0.08mol) and ammonium formate (6.1g, 0.096mol) Add DCM (100mL) solution into the reaction flask, then add TFA (4.5g, 0.04mol), tetraisopropyl titanate (4.5g, 0.0.16mol), 4A molecular sieve (10g) and metal complexes, pass into Hydrogen, reacted at 50°C for 24h, after the reaction was completed, neutralized with saturated aqueous sodium bicarbonate, extracted with DCM, passed through the column to remove the metal complex, and concentrated to obtain 14.1g of compound II (95% yield, enantiomer Selective S configuration:R configuration=99:1). 1 H-NMR (CDCl 3 ):δ2.80-3.03(m,2H),3.52(m,2H),3.79(m,4H),4.03(t,2H),4.87(m,1H),4.90-5.10(d,2H), 5.72(d,1H).
Embodiment 3
[0074]
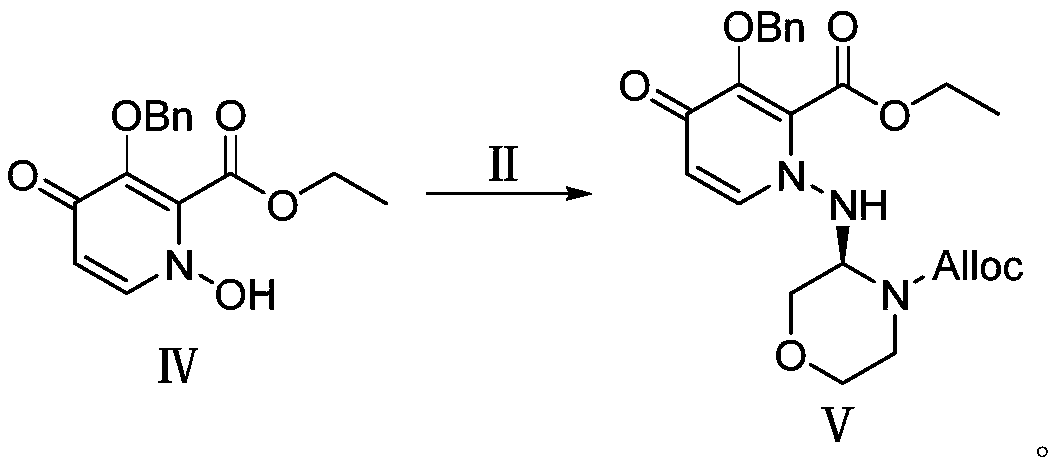
[0075] 3-(Benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid (24.6g, 0.1mol) and methanol (100mL), water (100mL) were added to the reaction flask, and hydroxylamine hydrochloride ( 34.8g, 0.5mol), reflux reaction for 10h, the reaction was cooled and then concentrated in vacuo, and methanol / water was used as eluent to pass through the column to obtain 23.5g of compound III (yield 90%). 1 H-NMR(DMSO):δ2.52(s,1H),4.95(s,2H),6.30(d,1H),7.00(d,1H),7.38-7.47(m,5H),10.00(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com