Artificially synthesized ferrihydrite and application method of artificially synthesized ferrihydrite in novel light-Fenton system
A technology of artificial synthesis and application method, which is applied in the direction of chemical instruments and methods, water treatment of special compounds, water pollutants, etc. It can solve the problems of narrow pH application range and large amount of iron sludge, so as to broaden the range of pH value and reduce iron pollution. Slime generation, speed-up effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 40.0g Fe(NO 3 ) 3 9H 2 O was dissolved in 500mL deionized water, and a KOH solution with a concentration of 1mol / L was added at a rate of 100mL / min, and the pH of the solution was adjusted to about 7.5 with 1mol / L KOH, and vigorously stirred for 1h. Centrifuge at a speed of 12 minutes for 12 minutes, discard the supernatant, wash the obtained solid with deionized water, shake well, and centrifuge at a speed of 4000 rpm for 12 minutes, discard the supernatant, repeat this 5 times and then store at 40°C After drying, the artificially synthesized ferrihydrite can be obtained.
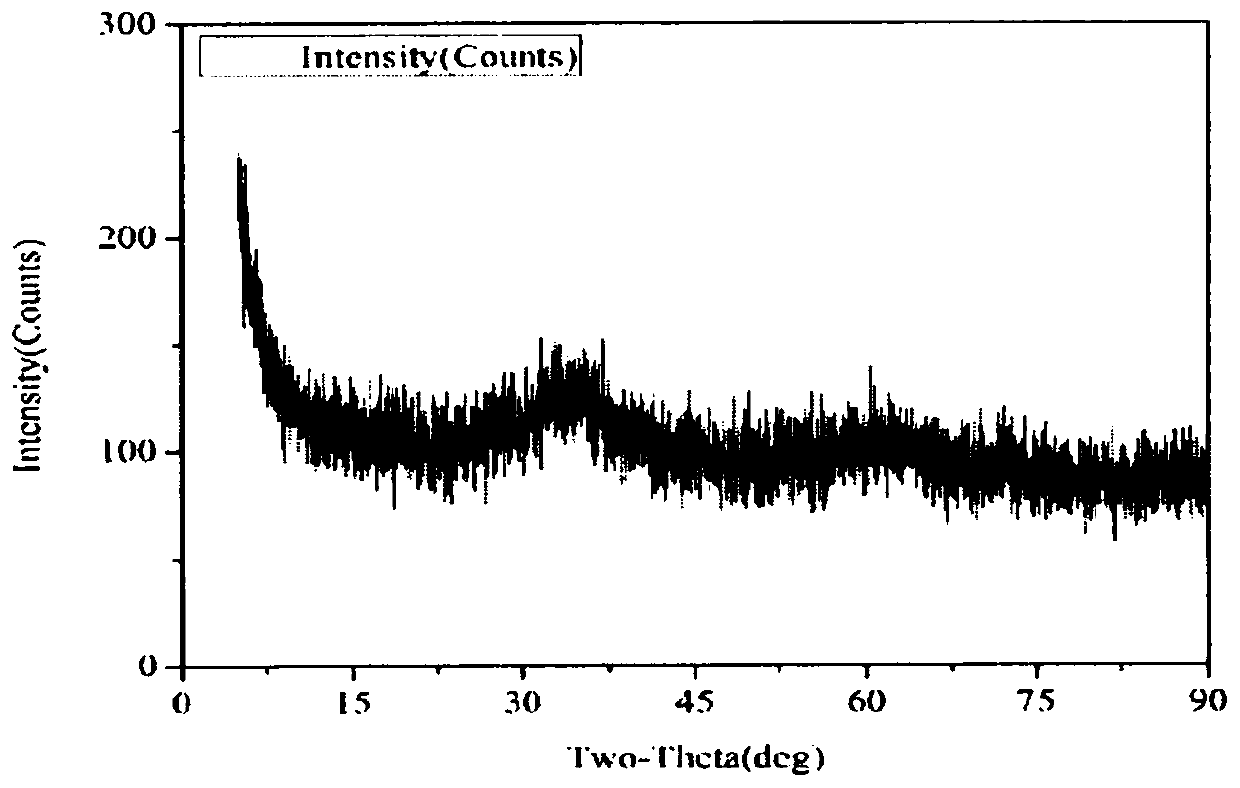
[0034] The crystal structure of the artificially synthesized ferrihydrite was analyzed by X-ray diffraction (XRD).
[0035] The measurement conditions are: XRD is carried out by tablet method at 40kV voltage and 100mA current, 2θ / θ coupled continuous scanning, 0.02° step width, and scanning speed of 10° / min. The result is as figure 1 shown. There are two broad peaks of weaker intensity i...
Embodiment 2
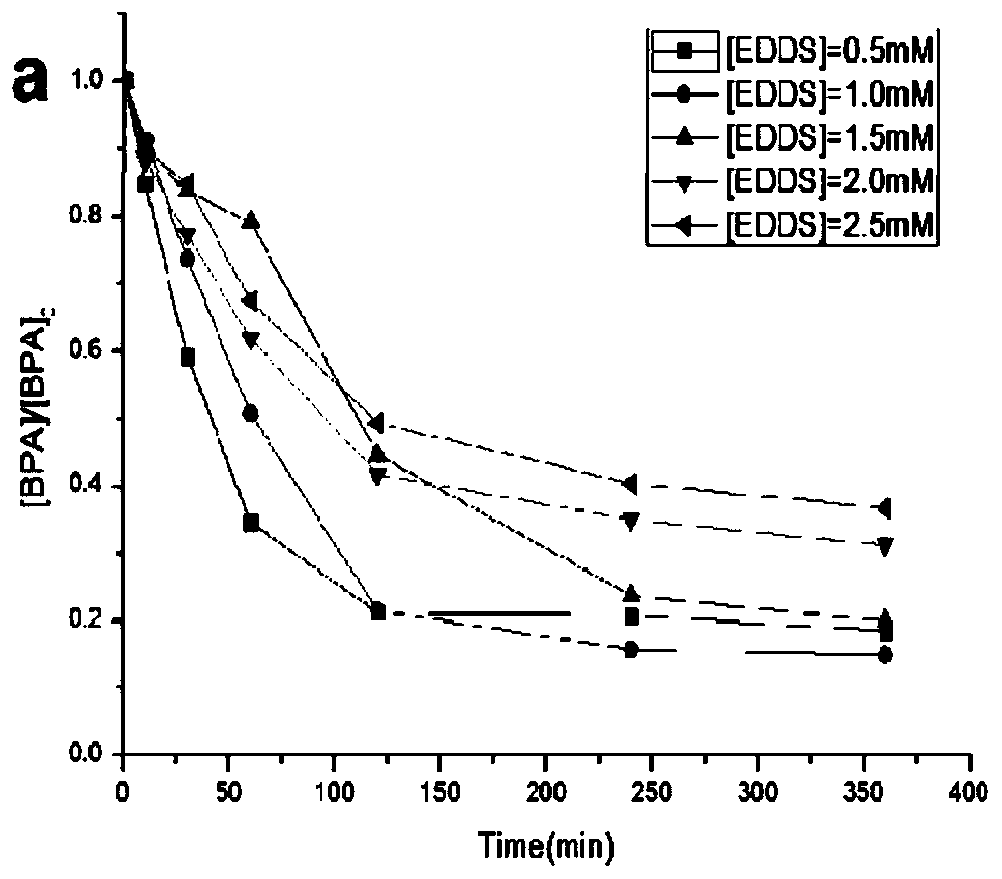
[0037] at 2.0 mmol L -1 Take 10mL of the BPA stock solution and place it in a 1L beaker, successively from 50mmol L -1 Pipette 10, 20, 30, 40, 50mL solutions from the EDDS stock solution into the beaker (the concentration of EDDS in the mixed system is 0.5, 1.0, 1.5, 2.0, 2.5mmol L -1 ), then add 0.6g ferrihydrite (prepared in Example 1) to the beaker, aerate it to fully complex, dilute to close to one liter, after it is fully mixed, adjust the pH to 7.0±0.1, then add 50 μL HO 2 o 2 Solution (30%, density 1.13g / cm 3 ), H 2 o2 The concentration of the solution in the mixed system is 0.5mmolL -1 , and then transferred to a 1000mL volumetric flask to constant volume, and then poured into a metal halide light catalytic reactor, and the timing was started when it was irradiated. At 0, 10, 30, 60, 120, 240, and 360 minutes, take 1.0mL water samples with a pipette gun for measurement, and the results are as follows figure 2 shown.
Embodiment 3
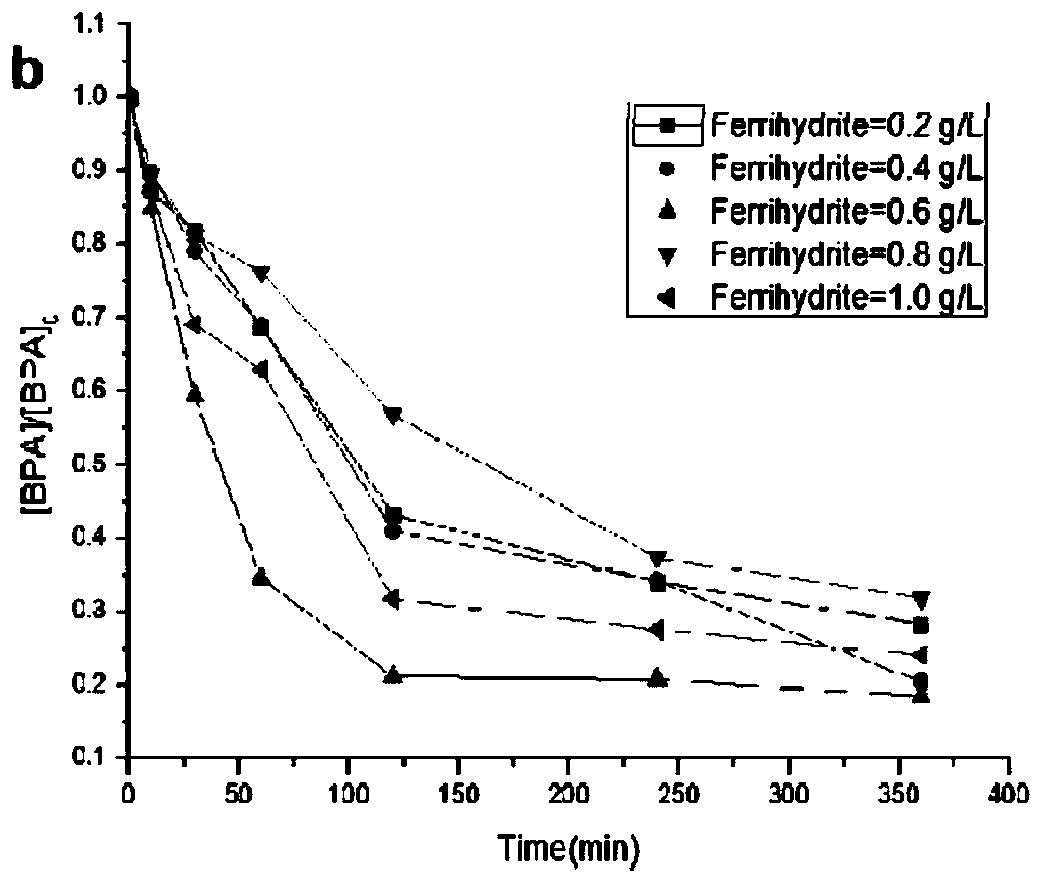
[0039] at 2.0 mmol L -1 Take 10mL of the BPA stock solution and place it in a 1L beaker, from 50mmol L -1 Pipette 20mL solution from the EDDS stock solution into the beaker (the concentration of EDDS in the mixed system is 1.0mmol L -1 ), then successively add 0.2, 0.4, 0.6, 0.8, 1.0g of ferrihydrite (prepared in Example 1) to the beaker, aerate to make it fully complexed, dilute to nearly one liter, and after it is fully mixed, Adjust the pH to 7.0 ± 0.1, then add 50 μL of HO 2 o 2 Solution (30%, density 1.13g / cm 3 ),H 2 o 2 The concentration of the solution in the mixed system is 0.5mmolL -1 , and then transferred to a 1000mL volumetric flask to constant volume, poured into a metal halide light catalytic reactor, and started timing when irradiated. At 0, 10, 30, 60, 120, 240, and 360 minutes, take 1.0mL water samples with a pipette gun for measurement, and the results are as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com