Preparation method of industrial grade lithium chloride
A lithium chloride, industrial-grade technology, applied in the field of preparation of industrial-grade lithium chloride, can solve the problems of strict control conditions, excessive barium ions and sodium ions, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
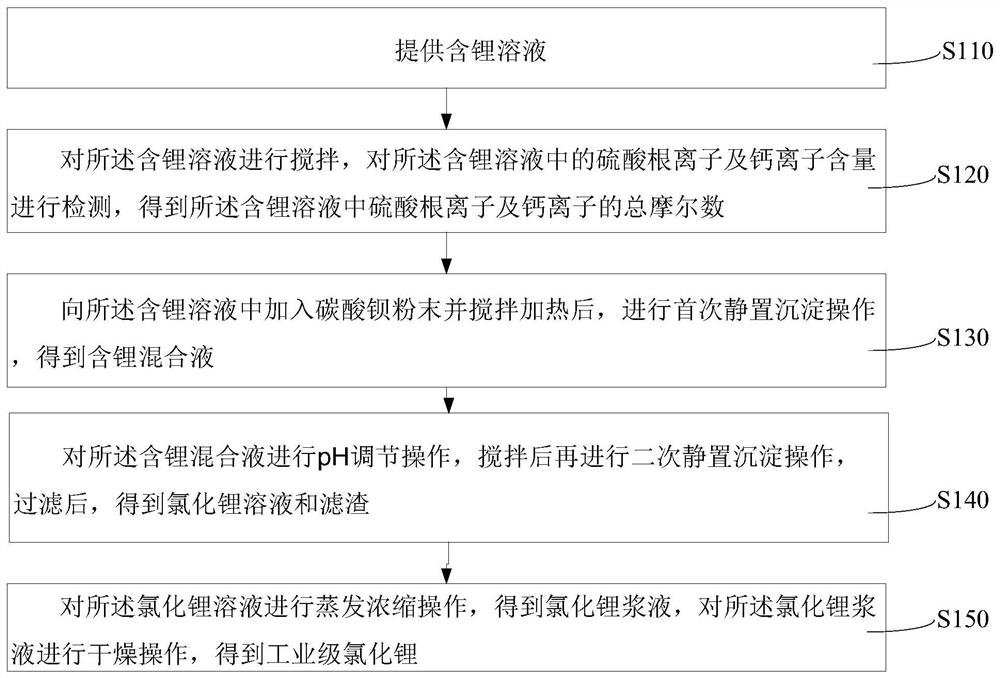
[0029] S110, providing a lithium-containing solution.
[0038] SO
[0045] In one embodiment, in the first standing precipitation operation, the time for standing and precipitation is 2h~4h. Reasonable
[0048] In one embodiment, in the pH adjustment operation of the lithium-containing mixed solution, the adjusted pH is 9 to 12. Reasonable
[0051] In one embodiment, in the secondary standing precipitation operation, the standing precipitation time is 4h~8h. understandably,
[0057] In one embodiment, in the drying operation, the drying operation adopts a centrifugal drying operation. understandably, by
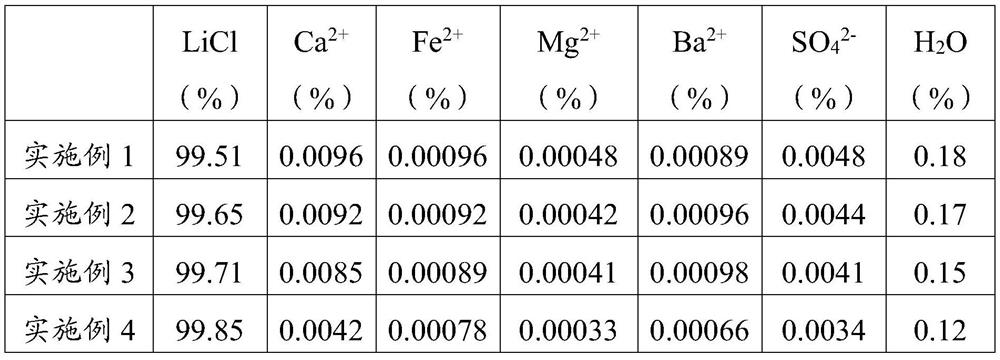
Embodiment 1
Embodiment 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com