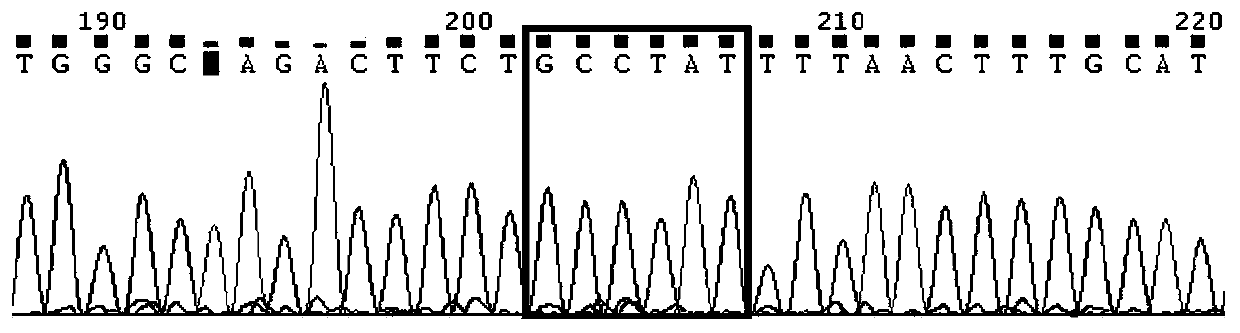
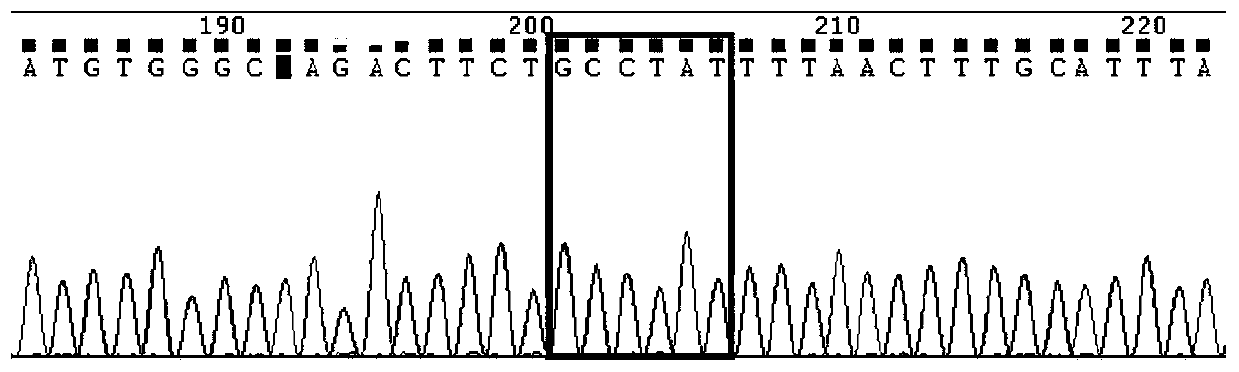
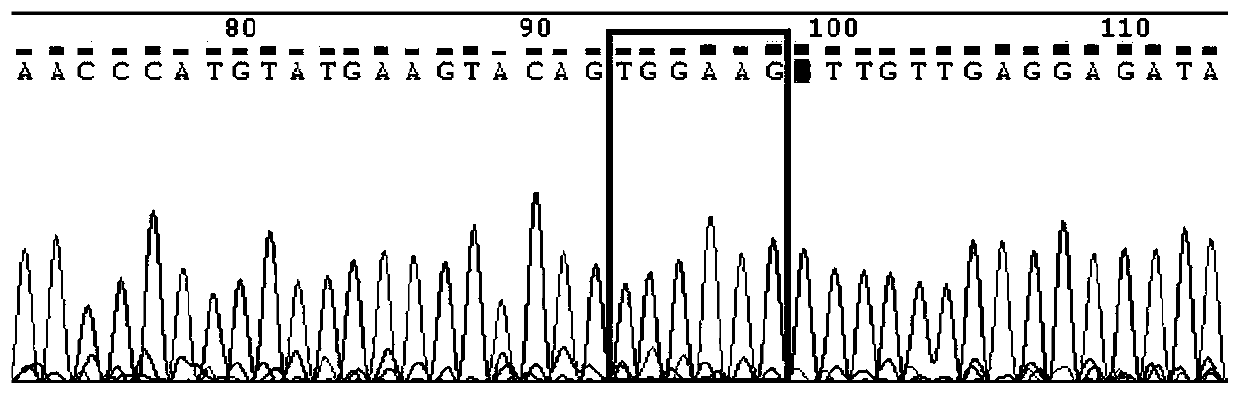
Kit for detecting imatinib targeted drug use related gene mutation
An imatinib target and kit technology, applied in the field of biomedicine, can solve the problems of high detection cost, low detection method sensitivity, less than 5% effective rate of chemotherapeutic drugs, etc., and achieves convenient and fast operation, good specificity and purification. Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The embodiment of the present invention provides a primer probe composition for detecting mutations of genes C-kit and PDGFRA related to imatinib-targeted drug use. The primer probe composition for detecting mutations of C-kit and PDGFRA genes includes Forward and reverse primers and fluorescent probes for detecting mutations in exon 9 of the C-kit gene, for detecting forward and reverse primers and fluorescent probes for exon 11 of the C-kit gene, for detecting C- Forward and reverse primers and fluorescent probes of the No. 13 exon of the kit gene are used to detect the No. 17 exon of the C-kit gene. Forward and reverse primers and fluorescent probes are used to detect the No. 12 exon of the PDGFRA gene Forward and reverse primers and fluorescent probes of the exon, and forward and reverse primers and fluorescent probes for detecting the No. 18 exon of the PDGFRA gene, wherein:
[0057] The forward primer of exon 9 of the C-kit gene is shown in SEQ ID NO.1 in the sequ...
Embodiment 2
[0077] The embodiment of the present invention provides a kit for detecting mutations of imatinib-targeted drug-related genes C-kit and PDGFRA. The kit includes the primer-probe composition provided in Example 1 of the present invention, each primer and fluorescent The probe concentration is 10 μmol / L, which can detect the mutations of exons 9, 11, 13 and 17 of C-kit gene and the mutations of exons 12 and 18 of PDGFRA gene .
[0078] The kit also includes PCR reaction solution, sequencing reaction solution, digestive enzyme system, PCR product purification reagent, negative quality control substance and positive quality control substance. The PCR reaction solution includes Mg-containing 2+ 5×PCR amplification buffer, 2.5mmol / L dNTPs and 5U / μL Taq DNA polymerase, the sequencing reaction solution includes 2.5×BigDye and 5×BigDye sequencing buffer.
[0079] The digestive enzyme system includes 1-4U / μL shrimp alkaline enzyme and 4-7U / μL exonuclease I.
[0080] On the basis of t...
Embodiment 3
[0083] Example 3, Detection of genes related to imatinib targeted drug
[0084] Patients with gastrointestinal stromal tumors who had a history of imatinib were selected as subjects for a retrospective study.
[0085] S1. Extracting sample genomic DNA
[0086]Applicable sample type: 10% neutral formalin-fixed paraffin-embedded gastrointestinal stromal tumor tissue section.
[0087] Sample requirements to be tested:
[0088] (1) Paraffin-embedded pathological section samples should be confirmed to contain tumor lesion cells. In order to ensure that the section tissue contains a sufficient proportion of tumor cells, one HE-stained slide of the same tissue should be added;
[0089] (2) Cut 5 pieces of 8-10 μm thick white slices from each sample, and the tumor cell area is larger than 5mm×5mm, slice serially, and seal them in a 1.5mL centrifuge tube;
[0090] (3) Paraffin-embedded pathological sections should not be stored at room temperature for more than 12 months. In order t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com