Daunorubicin-containing medicine, preparation method of daunorubicin-containing medicine, medical composition and application of daunorubicin-containing medicine
A daunorubicin and drug technology, applied in the field of medicine, can solve the problems of difficult connection, non-toxic degradation, impact, toxicity and self-design contradiction of transfection activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0156] According to the second aspect of the present application, there is also provided a method for preparing the above-mentioned daunorubicin-containing drug, which includes the following steps: providing any one of the above-mentioned nucleic acid nanoparticles; by means of physical connection and / or covalent connection The daunorubicin is mounted on the nucleic acid nanoparticles to obtain the daunorubicin-containing drug.
[0157] When physically linked, daunorubicin is usually intercalated between GC base pairs by physical intercalation. In the case of covalent linkage, daunorubicin usually undergoes a chemical reaction with the amino group outside the G ring to form a covalent linkage. The daunorubicin-containing drug prepared by the above-mentioned method can have better targeting after the target head is modified, can stably deliver the daunorubicin, and has high reliability.
[0158] In a preferred embodiment, the step of mounting daunorubicin by physical connectio...
Embodiment 1
[0180] 1. RNA and DNA nanoparticle carriers:
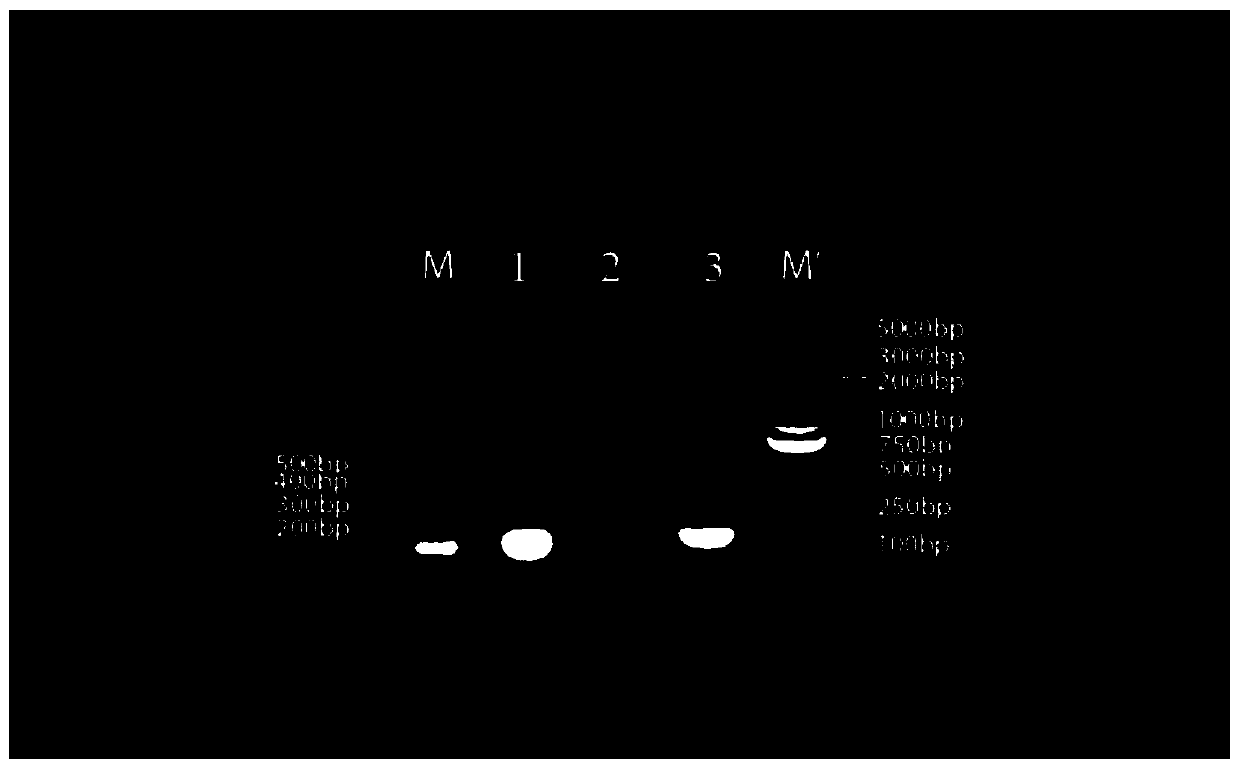
[0181] (1) The base sequences of the three polynucleotides that make up the RNA nanoparticles are shown in Table 1:
[0182] Table 1:
[0183]
[0184] (2) Three polynucleotide base sequences of DNA nanoparticles
[0185] The DNA uses the same sequence as the above RNA, except that T is substituted for U. Among them, the molecular weight of chain a is 8802.66, the molecular weight of chain b is 8280.33, and the molecular weight of chain c is 9605.2.
[0186] The a, b, and c strands of the above-mentioned RNA nanoparticles and DNA nanoparticles were all synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0187] 2. Self-assembly experimental steps:
[0188] (1) RNA or DNA single strands a, b, and c are simultaneously mixed and dissolved in DEPC water or TMS buffer at a molar ratio of 1:1:1;
[0189] (2) Heat the mixed solution to 80°C / 95°C (the RNA assembly temperature is 80°C, and the DNA assembly temperature is 95°...
Embodiment 2
[0200] 1. Seven groups of short-sequence RNA nanoparticle carriers:
[0201] (1) The base sequences of the three polynucleotides of the seven groups of RNA nanoparticles are shown in Table 2 to Table 8:
[0202] Table 2: R-1
[0203]
[0204] Table 3: R-2
[0205]
[0206] Table 4: R-3
[0207]
[0208] Table 5: R-4
[0209]
[0210] Table 6: R-5
[0211]
[0212] Table 7: R-6
[0213]
[0214] Table 8: R-7
[0215]
[0216] The single strands of the above seven groups of short-sequence RNA nanoparticle carriers were all synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0217] 2. Self-assembly experimental steps:
[0218] (1) RNA single strands a, b, and c are simultaneously mixed and dissolved in DEPC water or TMS buffer at a molar ratio of 1:1:1;
[0219] (2) Heat the mixed solution to 80°C, keep it for 5min and then cool down slowly to room temperature at a rate of 2°C / min;
[0220] (3) Load the product onto an 8% (m / v) non-denaturing P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com