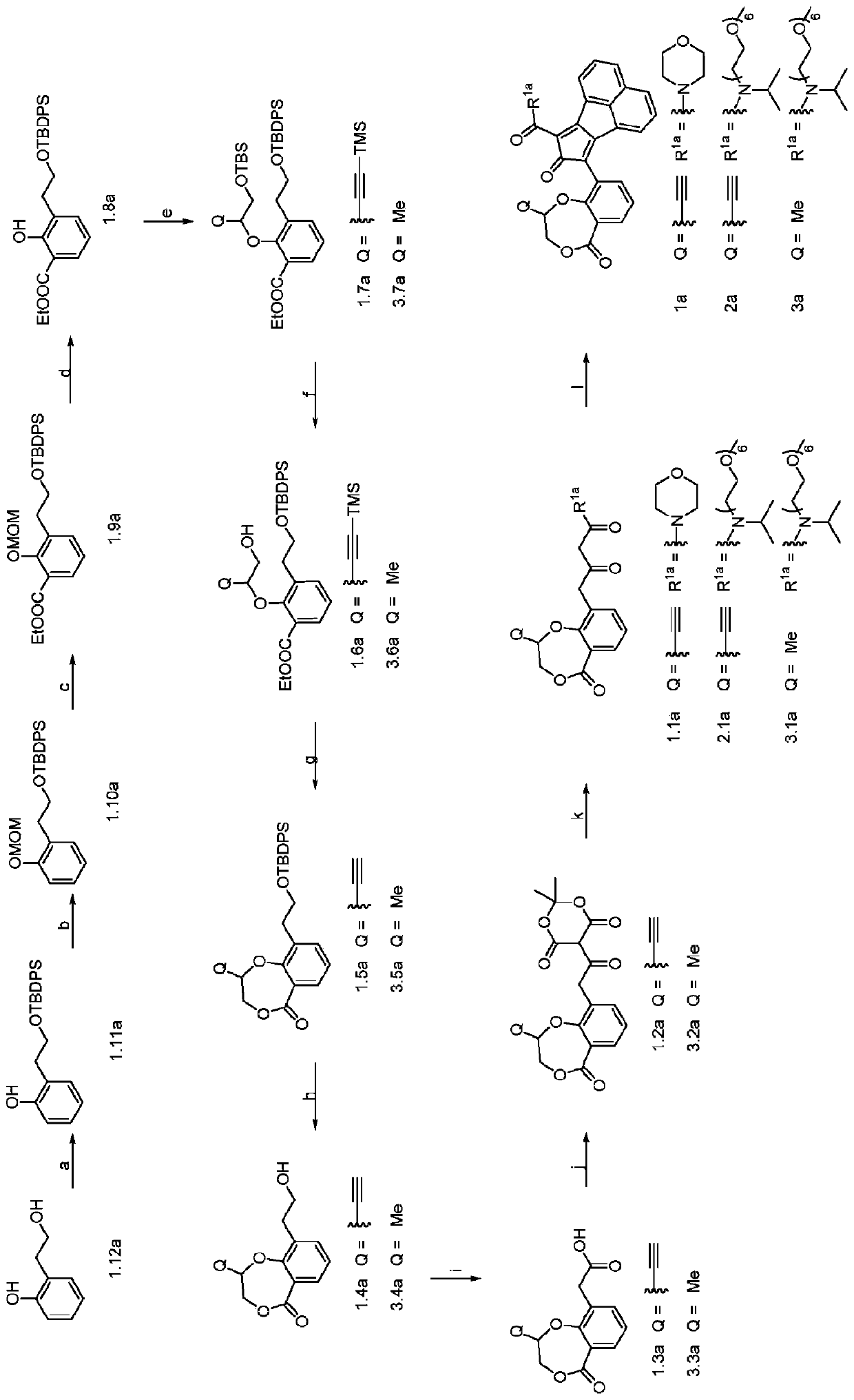
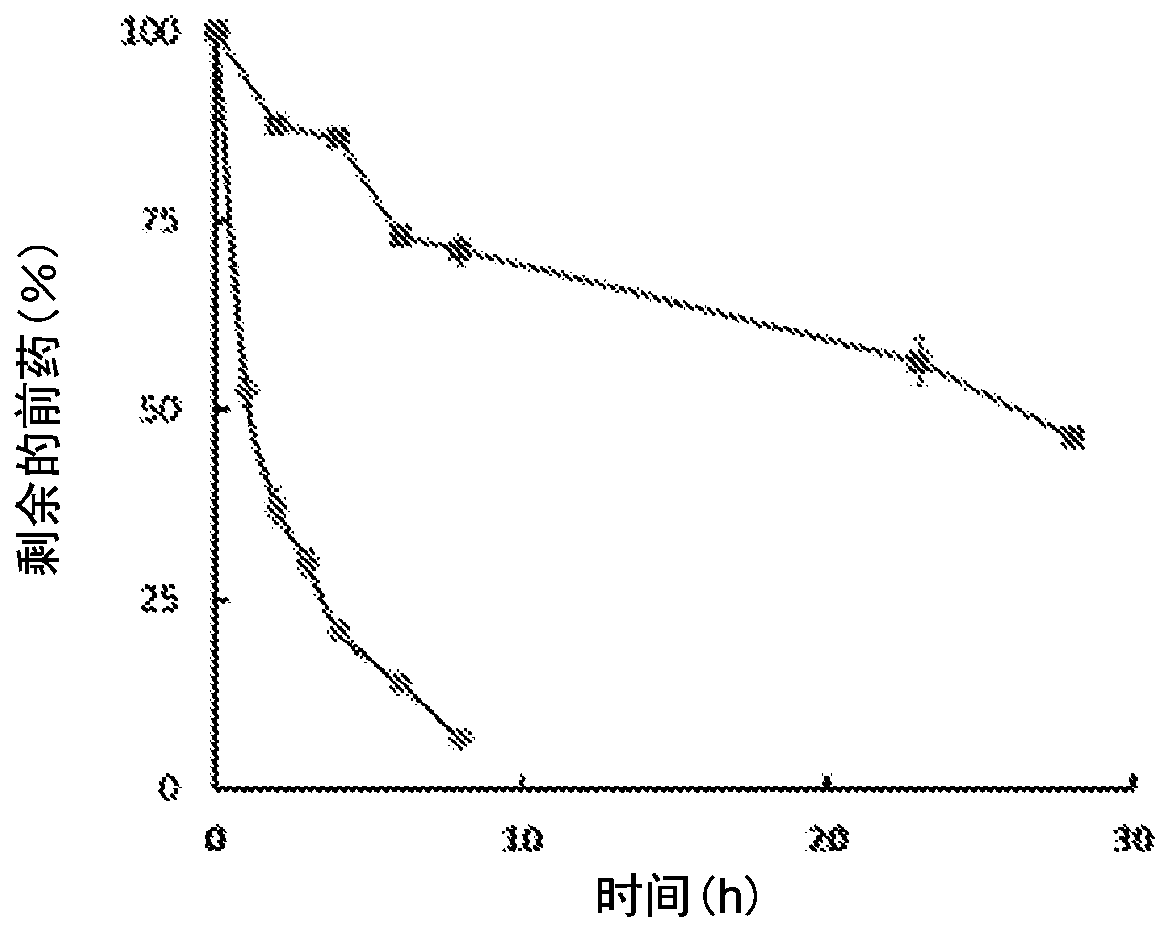
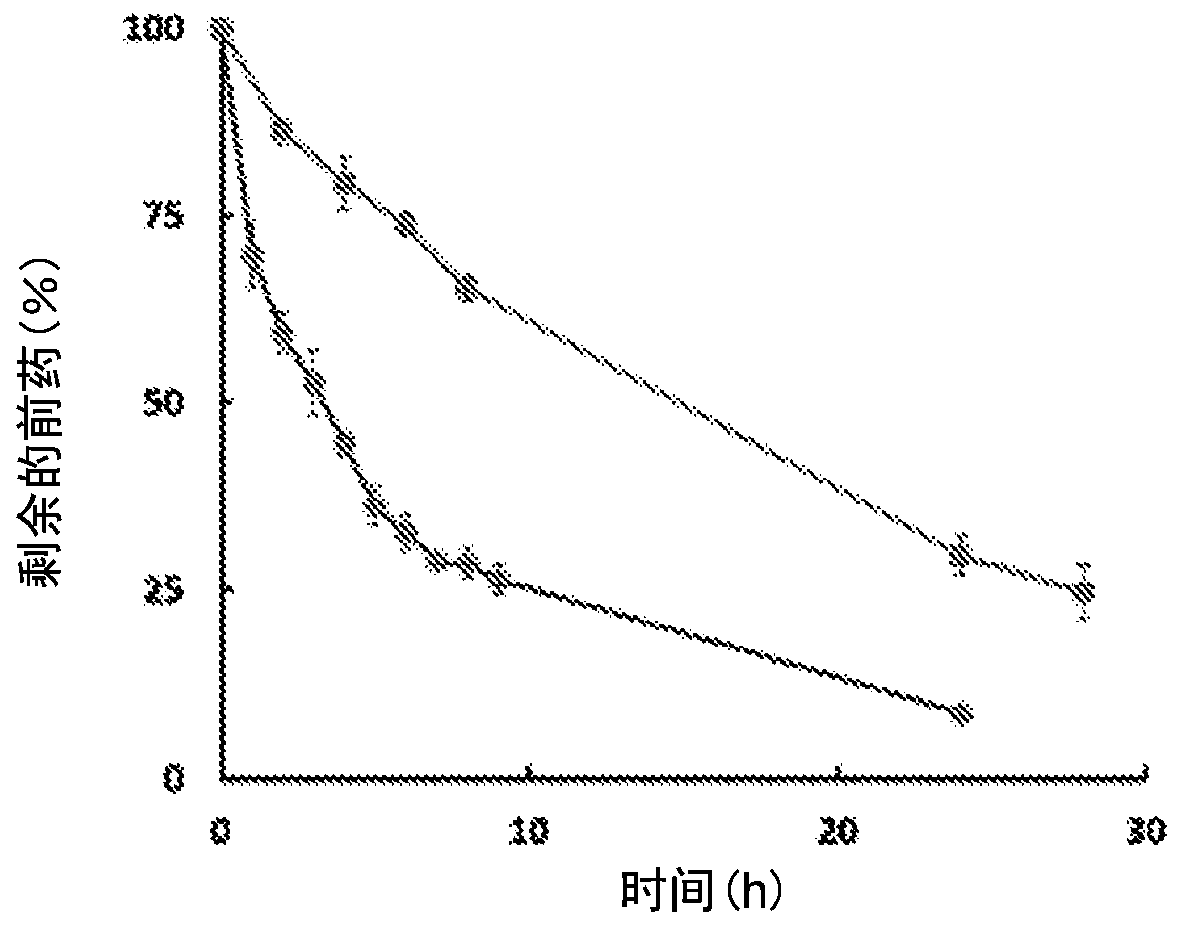
Enzyme-triggered carbon monoxide releasing molecules
A compound and selected technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, organic active ingredients, etc., can solve problems such as limited application and difficult control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Example 1: Synthesis of compound 1.11a
[0225]
[0226] N 2 , compound 1.12a (2.0 g, 14.5 mmol) and imidazole (1.5 g, 21.7 mmol) were dissolved in anhydrous dimethylformamide (DMF) (10 mL). Then, tert-butyldiphenylchlorosilane (TBDPSCl) (4.2 g, 15.2 mmol) was added dropwise at room temperature. The resulting mixture was further stirred at room temperature for 1 hour. Then, the mixture was poured into water and extracted with ethyl acetate (3 x 40 mL). The obtained organic layer was washed with anhydrous Na 2 SO 4 dry. After filtration and concentration, the residue was triturated with hexanes and the resulting precipitate was filtered to afford the title compound 1.11a. Yield: 90%. 1 H NMR (CDCl 3 ):δ8.16(s,1H),7.66(d,J=6.8Hz,4H),7.51–7.39(m,6H),7.24(t,J=7.2Hz,1H),7.05(d,J= 7.6Hz, 2H), 6.90(t, J=7.2Hz, 1H), 3.92(t, J=5.2Hz, 2H), 2.98(t, J=5.2Hz, 2H), 1.13(s, 9H). 13 C NMR (CDCl 3 ): δ155.8, 135.6, 132.0, 130.9, 130.1, 128.4, 127.9, 126.9, 120.4, 117.1, 66...
Embodiment 2
[0227] Embodiment 2: Synthesis of compound 1.10a
[0228]
[0229] To a solution of compound 1.11a (1.5 g, 4 mmol) in anhydrous DMF (25 mL) was added NaH (191 mg, 4.8 mmol) in portions at 0°C. The resulting mixture was then stirred at 0 °C for a further 0.5 h before methoxymethyl chloride (MOMCl) (480 mg, 6 mmol) was added dropwise. The resulting mixture was further stirred at room temperature for 4 hours. The reaction mixture was poured into ice water and extracted with ethyl acetate (3 x 40 mL). The resulting organic layer was washed with brine and washed with anhydrous Na 2 SO 4 dry. After filtration and concentration, the obtained residue was purified on a silica gel column to obtain compound 1.10a as a colorless oil (yield: 90%). 1 H NMR (CDCl 3 ): δ7.76–7.61(m,4H),7.54–7.37(m,6H),7.28–7.20(m,2H),7.17–7.06(m,1H),6.99(td,J=7.4,1.1Hz ,1H),5.14(s,2H),3.96(t,J=7.2Hz,2H),3.40(s,3H),3.05(t,J=7.2Hz,2H),1.13(s,9H). 13 C NMR (CDCl 3 ): δ155.4, 135.6, 134.0, 131.4, 129....
Embodiment 3
[0230] Embodiment 3: Synthesis of compound 1.9a
[0231]
[0232] To a solution of compound 1.10a (1.0 g, 2.4 mmol) in anhydrous tetrahydrofuran (THF) (30 mL) was added tetramethylethylenediamine (TMEDA) (417 mg, 3.6 mmol) at 0 °C, followed by 2 Next, n-butyllithium (n-BuLi) (1.8 mL, 3.6 mmol, 2M in hexane) was added dropwise. The resulting solution was stirred at room temperature for an additional 1 hour. The obtained brown solution was cooled to -78°C, then ethyl chloroformate (520 mg, 4.8 mmol) was added dropwise. A white precipitate formed immediately. The resulting mixture was stirred while slowly warming to room temperature (about 1 hour). The reaction mixture was then poured into saturated NH 4 Cl solution and extracted with ethyl acetate (3 x 40 mL). The combined organic layers were washed with anhydrous Na 2 SO 4 dry. After filtration and concentration, the obtained residue was purified on a silica gel column to obtain compound 1.9a as a colorless oil (yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com