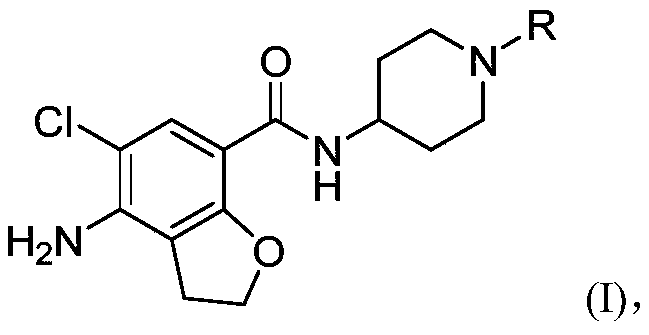
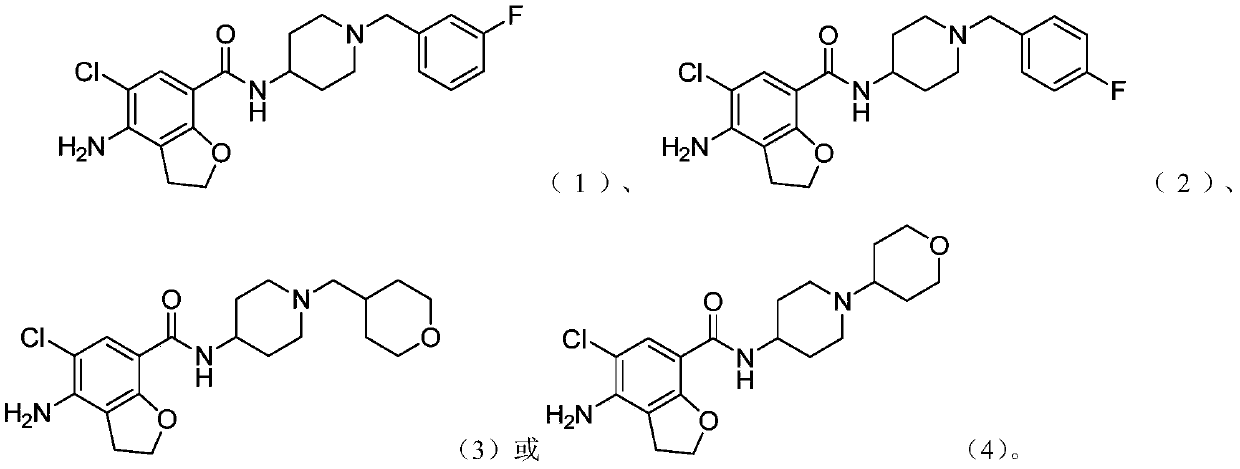
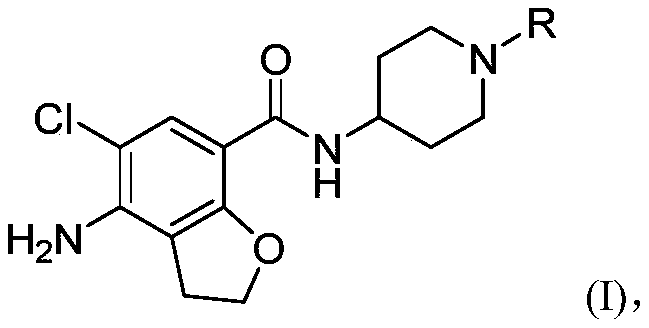
N-substituted piperidine amide derivative and application thereof
A technology of drugs and compounds, applied in the field of constipation-type irritable bowel syndrome, can solve problems such as poor clinical treatment effect, and achieve good pharmacokinetic properties, good bioavailability, and good safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0201] Example 1 Synthesis of 4-amino-5-chloro-N-(1-(3-fluorobenzyl)piperidin-4-yl)-2,3-dihydrobenzofuran-7-carboxamide
[0202]
[0203] Step 1) Synthesis of tert-butyl (1-(3-fluorobenzyl)piperidin-4-yl)carbamate
[0204] Weigh tert-butyl piperidin-4-yl carbamate (1.00g, 4.99mmol), dichloromethane (10mL), triethylamine (1.00g, 9.98mmol) and 1-(bromomethyl)-3-fluoro Benzene (1.13g, 5.99mmol) was placed in a 100mL single-neck flask, dichloromethane (10mL) was added, and the reaction was carried out at room temperature for 12 hours. The reaction solution was directly concentrated, and the residue was subjected to column chromatography (petroleum ether / ethyl acetate (v / v)= 5 / 1) The title compound was isolated as a white solid (1.09 g, 71%).
[0205] MS(ESI,pos.ion)m / z:309.25[M+H] + .
[0206] 1 H NMR(400MHz, CDCl 3 )δ(ppm) 7.32–7.27(m,1H),7.11–7.05(m,2H), 6.96(td,J=8.3,1.7Hz,1H), 4.49(s,1H), 3.50(s,3H) , 2.81 (d, J = 11.6 Hz, 2H), 2.13 (t, J = 10.6 Hz, 2H), 1.97-1.86 (m, 3H), 1.48 (s,...
Embodiment 2
[0215] Example 2 Synthesis of 4-amino-5-chloro-N-(1-(4-fluorobenzyl)piperidin-4-yl)-2,3-dihydrobenzofuran-7-carboxamide
[0216]
[0217] Step 1) Synthesis of tert-butyl (1-(4-fluorobenzyl)piperidin-4-yl)carbamate
[0218] Weigh tert-butyl piperidin-4-yl carbamate (1.00g, 4.99mmol), dichloromethane (10mL), triethylamine (1.00g, 9.98mmol) and 1-(bromomethyl)-4-fluoro Benzene (1.13g, 5.99mmol), add dichloromethane (10mL) to a 100mL single-necked flask, react at room temperature for 12h, directly concentrate the reaction solution, and subject the residue to column chromatography (petroleum ether / ethyl acetate (v / v) = 5 / 1) The title compound was isolated as a white solid (1.35 g, 88%).
[0219] MS(ESI,pos.ion)m / z:309.20[M+H] + .
[0220] Step 2) Synthesis of 1-(4-fluorobenzyl)piperidin-4-amine
[0221] Add dichloromethane (5mL) to a 50mL single-necked flask containing tert-butyl (1-(4-fluorobenzyl)piperidin-4-yl)carbamate (0.80g, 2.6mmol), then add hydrochloric acid and acetic acid Eth...
Embodiment 3
[0228] Example 3 4-Amino-5-chloro-N-(1-((tetrahydro-2H-pyran-4-yl)methyl)piperidin-4-yl)-2,3-dihydrobenzofuran Synthesis of -7-formamide
[0229]
[0230] Step 1) Synthesis of tert-butyl (1-((tetrahydro-2H-pyran-4-yl)methyl)piperidin-4-yl)carbamate
[0231] Sequentially combine tert-butyl N-(4-piperidinyl) carbamate (1.00g, 4.99mmol), 4-(bromomethyl)tetrahydropyran (1.16g, 6.48mmol) and potassium carbonate (5.52g, 39.9 mmol) was added to a 100mL single-neck flask, acetonitrile (20mL) was added, the reaction was heated to 90°C for 24 hours, the reaction was stopped, cooled to room temperature, the solvent was distilled off under reduced pressure, and the residue was separated and purified by column chromatography (petroleum ether / ethyl acetate Ester (v / v) = 2 / 1) gave the title compound as a white solid (0.68 g, 46%).
[0232] MS(ESI,pos.ion)m / z:299.30[M+H] + .
[0233] Step 2) Synthesis of 1-((tetrahydro-2H-pyran-4-yl)methyl)piperidin-4-amine hydrochloride
[0234] Weigh (1-((tetrah...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com