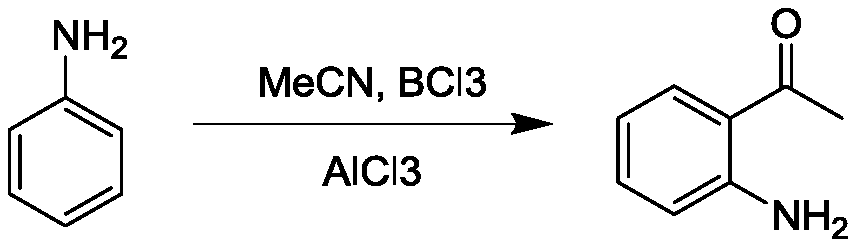
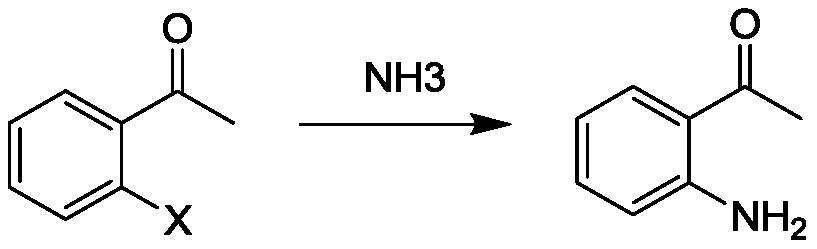
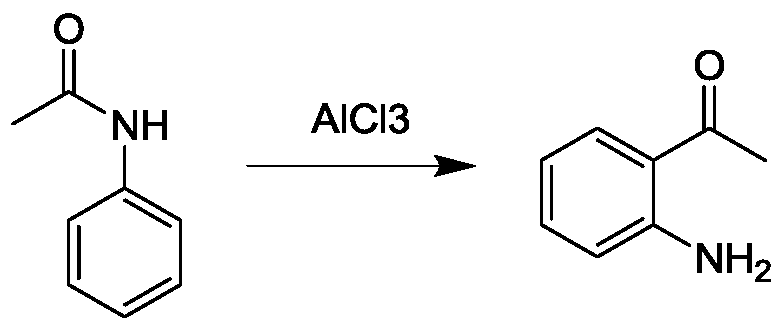
Preparation method of o-aminoacetophenone
A technology of o-aminoacetophenone and o-nitroethylbenzene, which is applied in the field of preparation of o-aminoacetophenone, achieves the effects of sufficient supply, cost saving, and simple preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add 80.0g o-nitroethylbenzene, 800mL DMF, 143.59g sodium chlorite, 63.59g acetic acid solution (50% content) into the three-necked flask and mix, then heat the mixed solution to 60-70°C for 20h, the reaction After cooling the final solution, quench the reaction with saturated sodium sulfite solution, then extract twice with ethyl acetate (1500mL*2), combine the ethyl acetate layer, wash once with saturated brine, and dry the organic phase with anhydrous sodium sulfate , concentrated, and the residue was distilled under reduced pressure to obtain 54.2 g of pure o-nitroacetophenone with a purity greater than 95%, with a yield of 62.01%.
[0049] Add 542mL of ethanol and 2.7g of palladium carbon to the obtained o-nitroacetophenone, at 50°C, 1MPa H 2 Hydrogenate under pressure for 12 hours, filter to remove palladium carbon, and rectify the filtrate to obtain o-aminoacetophenone with a purity greater than 99%.
Embodiment 2
[0051] Add 80.0g o-nitroethylbenzene, 800mL DMF, 264.97g sodium perbromate, 259.3g phosphoric acid solution (20% content) into the three-necked flask, then heat the mixed solution to 60-70°C for 20h, and put the reaction After the solution was cooled, the reaction was quenched with saturated sodium sulfite solution, extracted twice with ethyl acetate (1500mL*2), the combined ethyl acetate layer was washed once with saturated brine, and the organic phase was dried with anhydrous sodium sulfate. After concentration, the residue was distilled under reduced pressure to obtain 59.3 g of pure o-nitroacetophenone with a purity greater than 95%, with a yield of 67.85%.
[0052] Add 593mL of ethanol and 12g of Raney nickel to the o-nitroacetophenone in the above step, at 70°C, 1MPa H 2 Hydrogenate under pressure for 12 hours, remove Raney nickel by filtration, and rectify the filtrate to obtain o-aminoacetophenone with a purity greater than 99%.
Embodiment 3
[0054] Add 80.0g o-nitroethylbenzene, 800mL acetone, 264.97g sodium perbromate, 259.3g phosphoric acid solution (20% content) into the three-necked flask, then heat the mixed solution to 60-70°C for 20h, and the reaction After the solution was cooled, the reaction was quenched with saturated sodium sulfite solution, extracted twice with ethyl acetate (1500mL*2), the combined ethyl acetate layer was washed once with saturated brine, and the organic phase was dried with anhydrous sodium sulfate. After concentration, the residue was distilled under reduced pressure to obtain 48.5 g of pure o-nitroacetophenone with a purity greater than 95%, with a yield of 55.4%.
[0055] Add 485mL of methanol and 2.4g of palladium carbon to the o-nitroacetophenone in the above step, at 40°C, 1MPa H 2 Hydrogenate under pressure for 12 hours, filter to remove palladium carbon, and rectify the filtrate to obtain o-aminoacetophenone with a purity greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com