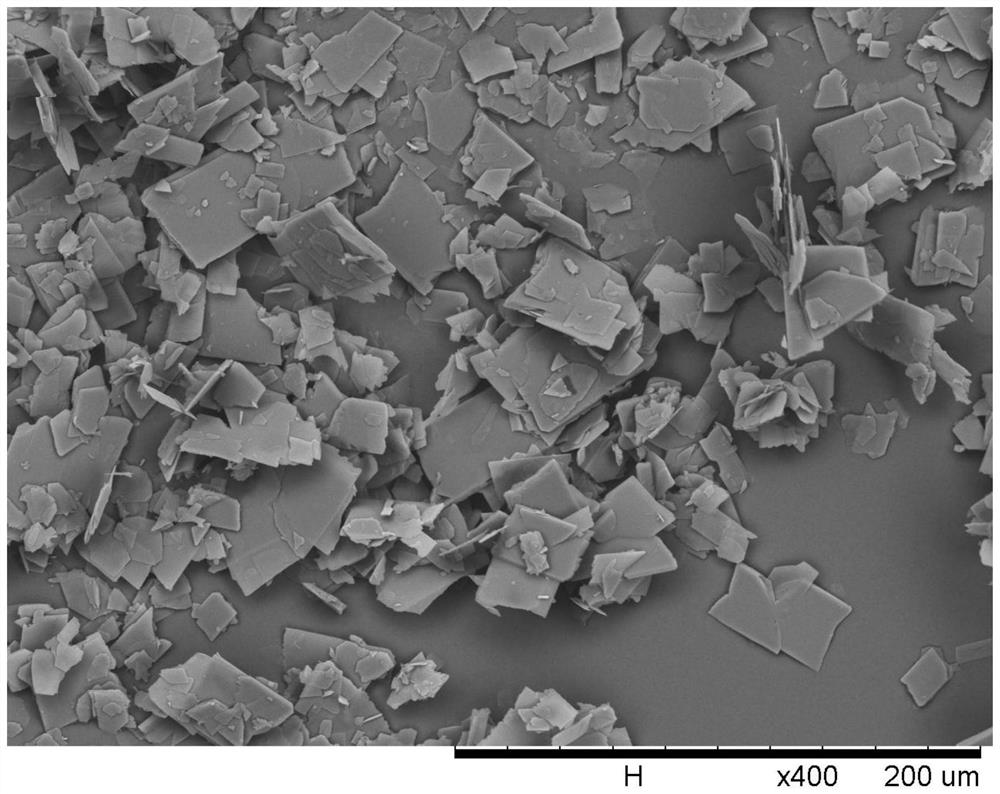
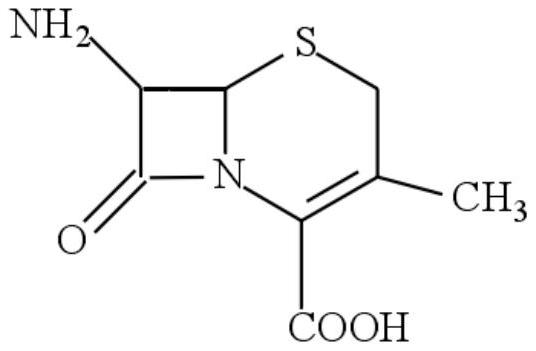
A kind of cephalexin crystallization mother liquor recovery method
A recovery method, the technology of cephalexin, which is applied in the fields of chemical industry and medicine, can solve the problems of low product purity, high cost, and low yield, and achieve the effects of high product purity and yield, efficient recovery, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Under stirring at 25°C, add ammonia solution to 1L of cephalexin crystallization mother liquor, keep the pH value at 7.65, add 90 g of penicillin G acylase, carry out the cleavage reaction of cephalexin for 15 minutes to obtain a lysate, and filter the lyse with suction Afterwards, 980 mL of lysed liquid was obtained, and the conversion rate of cephalexin was 99.4%;
[0042] (2) Under stirring, add sulfuric acid to the lysed liquid obtained in step (1), adjust the pH to 6.7, and cool to 5°C at a cooling rate of 5°C / h to precipitate the phenylglycine in the lysed liquid Precipitated, obtained 960mL clear liquid and phenylglycine solid after suction filtration;
[0043] (3) Under stirring, add sulfuric acid to the clear liquid separated in step (2) at a rate of 14.4mL / h, adjust the pH value to 3.9, and heat up to 30°C, then perform suction filtration, wash and dry, The 7-aminodesacetoxycephalosporanic acid product was obtained.
[0044] The results show that based o...
Embodiment 2
[0046] (1) Under stirring at 40° C. and 300 rpm, sodium hydroxide solution is added to 1 L of cephalexin crystallization mother liquor, and the pH value is maintained at 8.0, 70 g of penicillin G acylase is added, and the cleavage reaction of cephalexin is carried out for 20 minutes to obtain a lysate. After the lysate was centrifuged, 990 mL of the lysate clear liquid was obtained, and the conversion rate of cephalexin was 99.5%;
[0047] (2) Under the action of stirring at 300rpm, hydrochloric acid is added to the lysed clear liquid obtained in step (1), the pH value is adjusted to 6.5, and cooled to 10° C. at a cooling rate of 10° C. / h to make the benzene in the lysed clear liquid Glycine was precipitated, and after centrifugation, 970 mL of clear liquid and phenylglycine solid were obtained;
[0048] (3) Under 300rpm stirring, add hydrochloric acid to the clear liquid separated in step (2) at a rate of 20mL / h, adjust the pH value to 3.7, and heat up to 35°C, then centrifug...
Embodiment 3
[0051] (1) Under stirring at 15°C and 220rpm, potassium hydroxide solution was added to 1L of cephalexin crystallization mother liquor, and the pH value was maintained at 7.8, 60 g of penicillin G acylase was added, and the cleavage reaction of cephalexin was carried out for 45 minutes to obtain a lysate. After the lysate was filtered under normal pressure, 995 mL of the lysed clear liquid was obtained, and the conversion rate of cephalexin was 99.1%;
[0052] (2) Under the action of stirring at 220rpm, phosphoric acid is added to the lysed clear liquid obtained in step (1), the pH value is adjusted to 7, and cooled to 7° C. at a cooling rate of 4° C. / h, so that the benzene in the lysed clear liquid is Glycine was precipitated out, and 975mL of clear liquid and phenylglycine solid were obtained after normal pressure filtration;
[0053] (3) Under the action of stirring at 220rpm, add phosphoric acid to the clear liquid separated in step (2) at a rate of 30mL / h, adjust the pH v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com