Antibody conjugates of immune-modulatory compounds and uses thereof
A technology of immune regulation and conjugates, applied in the direction of antibodies, immunoglobulins, anti-growth factor immunoglobulins, etc., which can solve the problems of lack of treatment for fibrotic diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] In certain embodiments, the compounds disclosed herein have 2 Some or all of the H atoms substituted 1 H atom. Synthetic methods of deuterium-containing compounds are known in the art, and include the following synthetic methods only as non-limiting examples.
[0074] Deuterium substituted compounds were synthesized using various methods such as those described in: Dean, Dennis C.; Editor. Recent Advances in the Synthesis and Applications of Radiolabeled Compounds for Drug Discovery and Development. [In: Curr., Pharm. Des., 2000 6(10)] 2000, p. 110; George W.; Varma, Rajender S. The Synthesis of Radiolabeled Compounds via Organometallic Intermediates, Tetrahedron, 1989, 45(21), 6601-21; and Evans, E. Anthony. Synthesis of radiolabeled compounds, J. Radioanal. Chem., 1981, 64(1-2), 9-32.
[0075] Deuterated starting materials are readily available and subjected to the synthetic methods described herein to provide synthesis of deuterium-containing compounds. A large n...
Embodiment 1
[0625] Synthesis of immunomodulatory compounds, linker payloads and conjugates
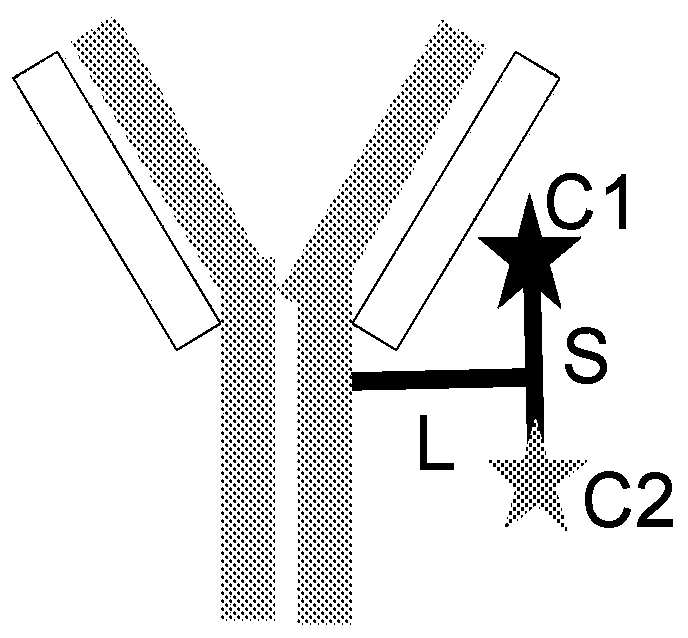
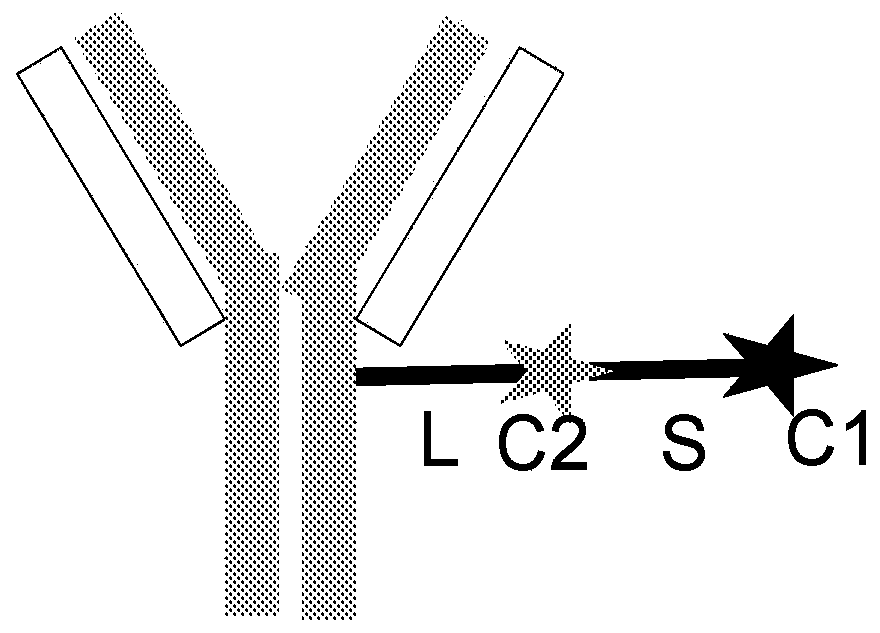
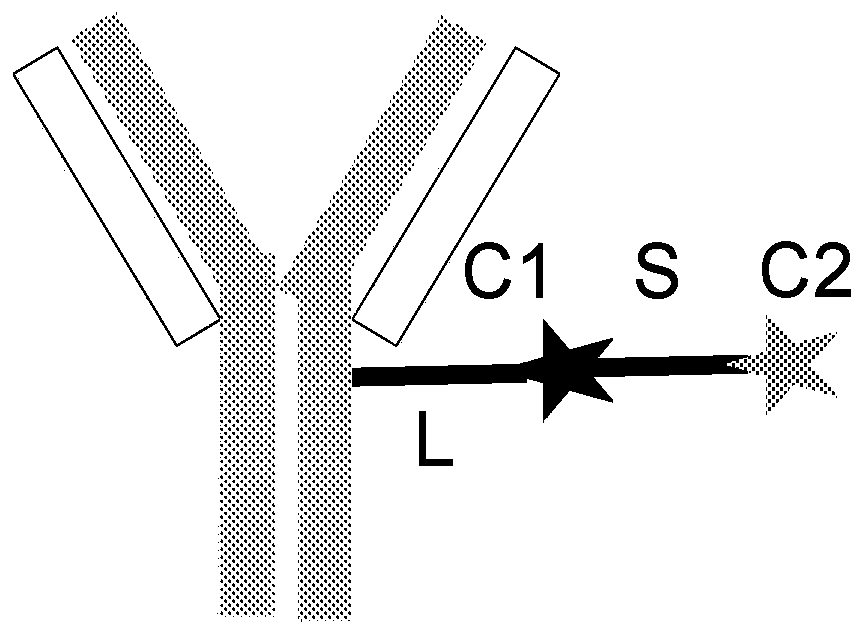
[0626] Linkers with immunomodulatory compounds such as PI3K inhibitors, calcineurin inhibitors, mTOR inhibitors, BTK inhibitors, JAK inhibitors, CRAC inhibitors, PARP1 antagonists, PPARg agonists, Kv1.3 antagonists, KCa3. 1 Antagonist, PP2A agonist, IRAK4 inhibitor, MYD88 inhibitor, BCL-2 antagonist, A2ar agonist, TLR7 antagonist, c-KIT kinase inhibitor, KCA3.1 agonist, TGFβR1 inhibitor, TGFβR2 inhibitor , ACC antagonist, ASK1 antagonist, GLI1 inhibitor, TNKS antagonist or TNIK antagonist link. Linker to Immunomodulatory Compound A linker-immunomodulatory compound (LP) was prepared. Subsequently, LP is conjugated to the antibody construct. Such as antibodies to form antibody constructs immunomodulatory compound conjugates or conjugates.
[0627] Inhibitors of TGFβR2
Embodiment 11
[0628] Example 1.1 Synthesis of (S)-N1-(4-(5-amino-6-((4-morpholinopyridin-3-yl)carbamoyl)pyrazin-2-yl)benzyl)-2- (6-(4-((2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)methyl)-cyclohexane-1-carboxamido)hexanoylamino)- N5-(2-(3-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl )pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-3-oxopropoxy)ethyl)glutaramide (compound 1-1 )
[0629]
[0630] Step A: Preparation of Int 1B-1
[0631]
[0632] HATU (3.54 g, 9.36 mmol) was added to a solution of 1.64 g (7.5 mmol) 3-amino-6-bromopyrazine-2-carboxylic acid in 25 mL DMF. The reaction was stirred for 5 minutes, then 2.5 mL (22.5 mmol) of N-methylmorpholine and 1.68 g (9.36 mmol) of 4-morpholinopyridin-3-amine were added. The reaction mixture was stirred for 16 h, then washed with 10 mL of saturated NH 4 The Cl solution followed by 10 mL of water was quenched. The mixture was extracted 3 times with EtOAc; the combined organics were washed with brine, then wash...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com